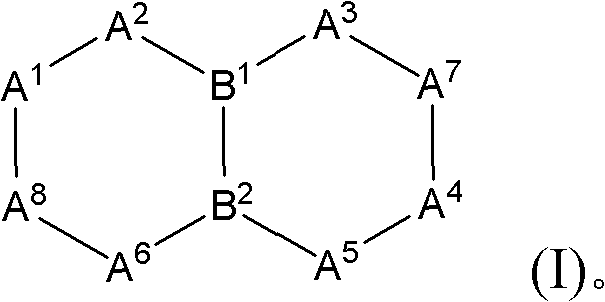
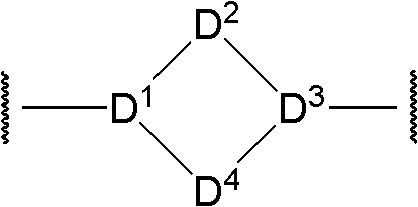
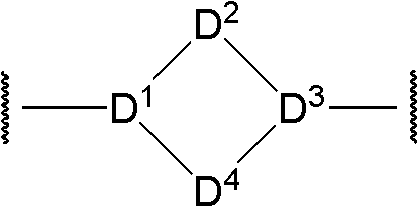
Bicyclic aryl sphingosine 1-phosphate analogs
A heteroaryl, A1-A6 technology, applied in the field of bicyclic aryl sphingosine 1-phosphate analogs, can solve the problem of increasing cell migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] Example 1: cis-4-tert-butylcyclohexyl methanesulfonate
[0165]
[0166] Cis-4-t-butylcyclohexanol (6.0 g, 38.5 mmol, 1.0 eq.) was dissolved in dichloromethane (10 mL). Then methanesulfonic anhydride (8.03 g, 46.2 mmol, 1.1 eq.) was slowly added to the mixture at 0 °C. Then triethylamine (6.4 mL, 46.2 mmol, 1.5 eq.) was added to the mixture, and the mixture was stirred at room temperature for 3 h. The mixture was extracted with dichloromethane, and then the organic layer was concentrated to obtain a white powder of the product (8.0 g, yield: 90%). The product was used in the next step without further purification. 1 HNMR (400MHz, CDCl 3 ) δ 4.99-4.98 (m, 1H), 3.02 (s, 3H), 2.14-2.12 (m, 2H), 1.65-1.28 (m, 7H), 0.84 (s, 9H).
Embodiment 2
[0167] Example 2: 2-bromo-6-(trans-4-tert-butylcyclohexyloxy)naphthalene
[0168]
[0169] 6-bromonaphthalen-2-ol (CAS no. 15231-91-1) (3.0 g, 14.8 mmol, 1.0 eq.) was dissolved in a mixture of t-butanol / 2-butanone (4 mL / 2 mL). Cesium carbonate (12 g, 37.2 mmol, 2.5 eq.) was then added to the mixture, and the mixture was stirred at 110° C. for 10 min. Then trans-4-tert-butylcyclohexyl methanesulfonate (3.48 g, 16.2 mmol, 1.1 eq.) was added to the mixture. The suspension was stirred at 110 °C for 15 h under nitrogen atmosphere. The reaction mixture was extracted with ethyl acetate, and the organic layer was purified by silica gel column chromatography using petroleum ether as an eluent to give 2-bromo-6-(trans-4-tert-butylcyclohexyloxy)naphthalene (1.7 g, yield: 32%). ESI-MS: 361.0 (M+H) + . 1 HNMR (400MHz, CDCl 3 )δ7.89(s, 1H), 7.63(d, 1H), 7.56(d, 1H), 7.47(d, 1H), 7.15-7.11(m, 2H), 4.26-4.24(m, 1H), 2.27 -2.25 (m, 2H), 1.89-1.87 (m, 2H), 1.45-1.09 (m, 5H), 0.89 (s, ...
Embodiment 3
[0170] Example 3: 6-(trans-4-tert-butylcyclohexyloxy)-2-naphthaldehyde
[0171]
[0172] 2-Bromo-6-(trans-4-tert-butylcyclohexyloxy)naphthalene (2.249 g, 6.25 mmol, 1.0 eq.) was dissolved in THF (10 mL) under nitrogen atmosphere. The mixture was then cooled to -78 °C, and a solution of n-BuLi in THF (2.5M, 7.5 mL, 18.8 mmol, 3.0 eq.) was added dropwise to the mixture. The mixture was stirred at -78°C for 15 min. Then DMF (2.4 mL, 31.2 mmol, 5.0 eq.) was added to the mixture and stirred at -78 °C for 1 h. When the reaction was complete, 1M HCl was added to adjust the pH to 6. The mixture was extracted with EtOAc, then the organic layer was concentrated and purified by silica gel chromatography using petroleum ether / ethyl acetate (10 / 1) as eluent to give 6-(trans-4-tert Butylcyclohexyloxy)-2-naphthaldehyde as a white solid (1.16 g, 60%). EDI-MS: 311.1(M+H) + . 1 H NMR (400MHz, CDCl 3 )δ10.08(s, 1H), 8.24(s, 1H), 7.92-7.87(m, 2H), 7.77(d, 1H), 7.22-7.19(m, 2H), 4.42-4.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com