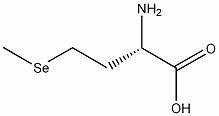
Environmentally-friendly method for synthesizing selenomethionine
An environmentally friendly technology of selenomethionine, applied in the direction of organic chemistry, can solve the problems of low process yield and environmental pollution, achieve high product purity, high process yield, and improve the effect of health and environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthesis of embodiment one L-selenomethionine
[0049] Follow the steps:
[0050] one. Synthetic tert-butoxycarbonyl-homoserine (hereinafter referred to as "Boc-HomoSer-OH") belongs to the compound of general formula (2), and its structural formula is as follows:
[0051]
[0052] Dissolve 380g of tert-butoxycarbonyl-L-aspartic acid 4-methyl ester in 3600ml of THF, cool to 0°C with dry ice, add to the reaction kettle, and add NaBH in batches 4 , control the temperature at 5°C to 10°C, and react overnight at 5°C to 20°C after the addition is complete. Add methanol (2000ml) to the reaction solution until no bubbling, stir for ten minutes, concentrate under reduced pressure to remove half of the volume, adjust the pH to 7-8 with 50% phosphoric acid, continue to concentrate the material to dryness to obtain a large amount of viscous, Add 600ml of water, and stir while scraping. After a large amount of viscous matter dissolves, the resulting aqueous layer is extr...
Embodiment 2
[0068] Example 2 Synthesis of D-selenomethionine
[0069] Follow the steps:
[0070] one. Synthesize tert-butoxycarbonyl-D-homoserine, which belongs to the compound of general formula (2), and its structural formula is as follows:
[0071]
[0072] Dissolve 38g of Boc-D-aspartic acid 4-methyl ester in THF (360ml), cool to 0°C with dry ice, add to the reaction kettle, and add NaBH in batches 4 , Control the temperature at 5°C to 10°C. After the addition, react overnight at 5°C to 20°C. Add methanol (200ml) to the reaction solution until it stops bubbling, and stir for ten minutes. After concentrating half of the volume, adjust the pH to 7-8 with 50% phosphoric acid, and continue concentrating to dryness to obtain a large amount of viscous. Add 60ml of water and stir while scraping. After a large amount of viscous matter dissolves, the resulting aqueous layer is extracted with DCM (20 ml each time, repeated 3 times). The ethyl acetate solution obtained by ester extracti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com