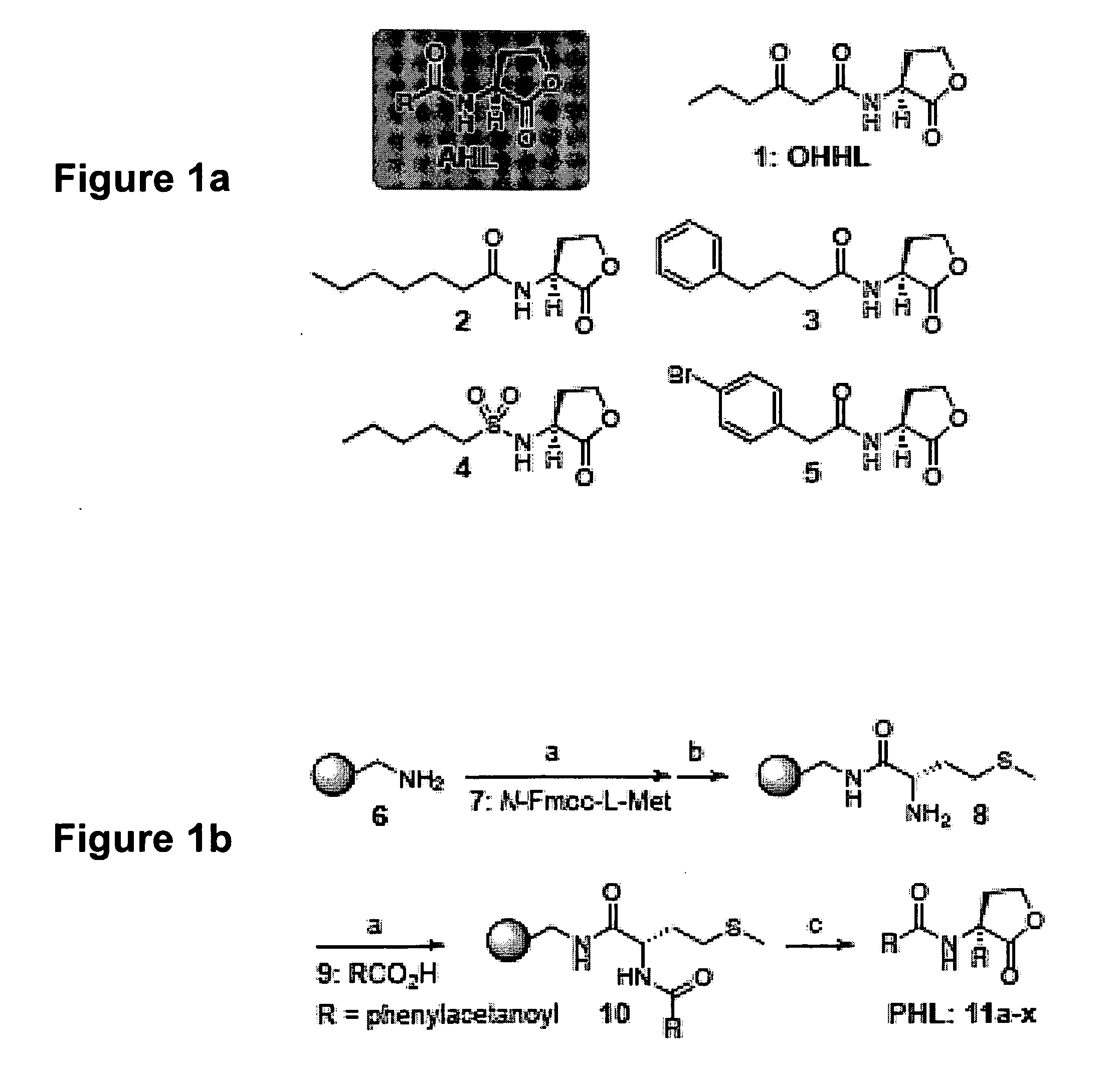
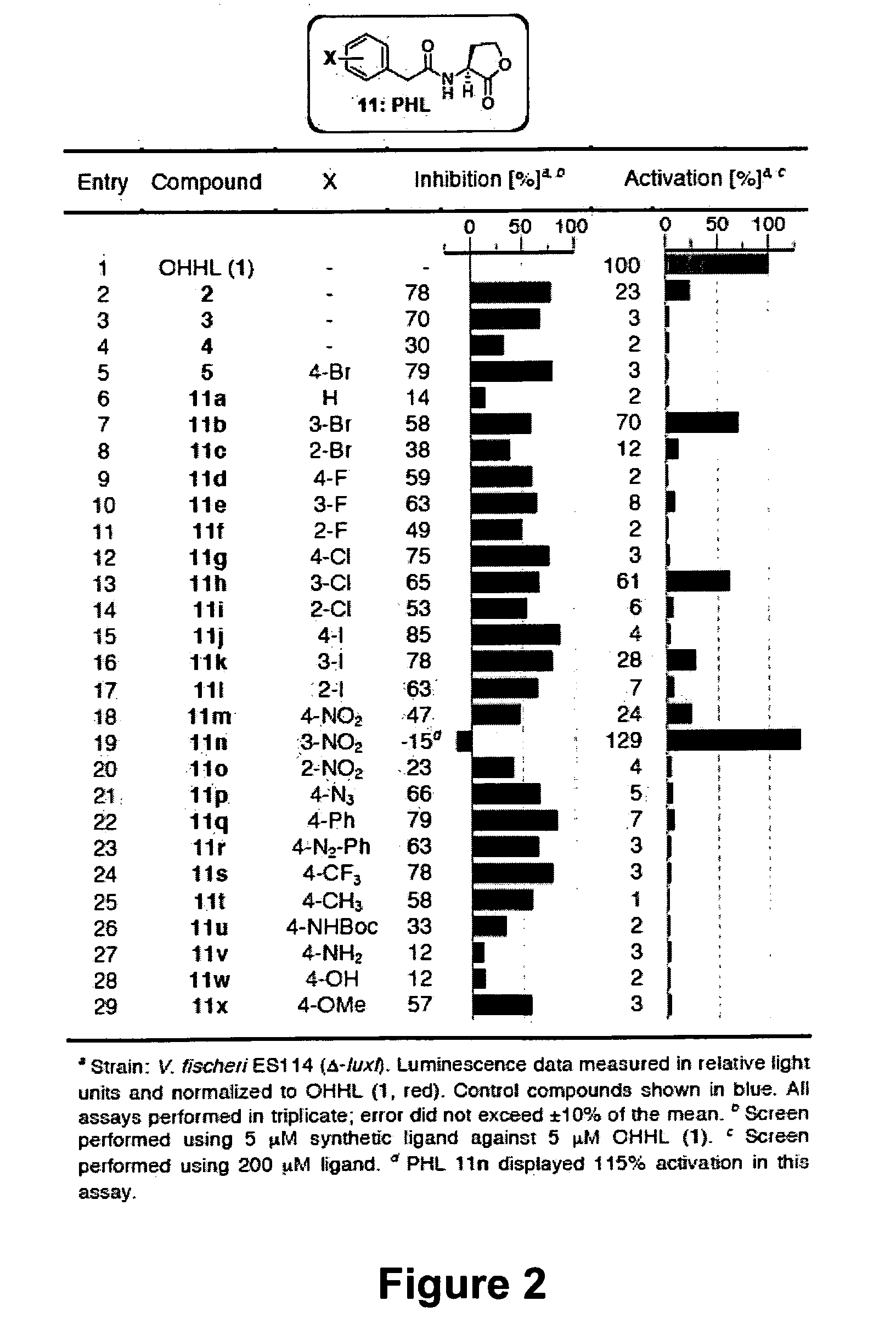
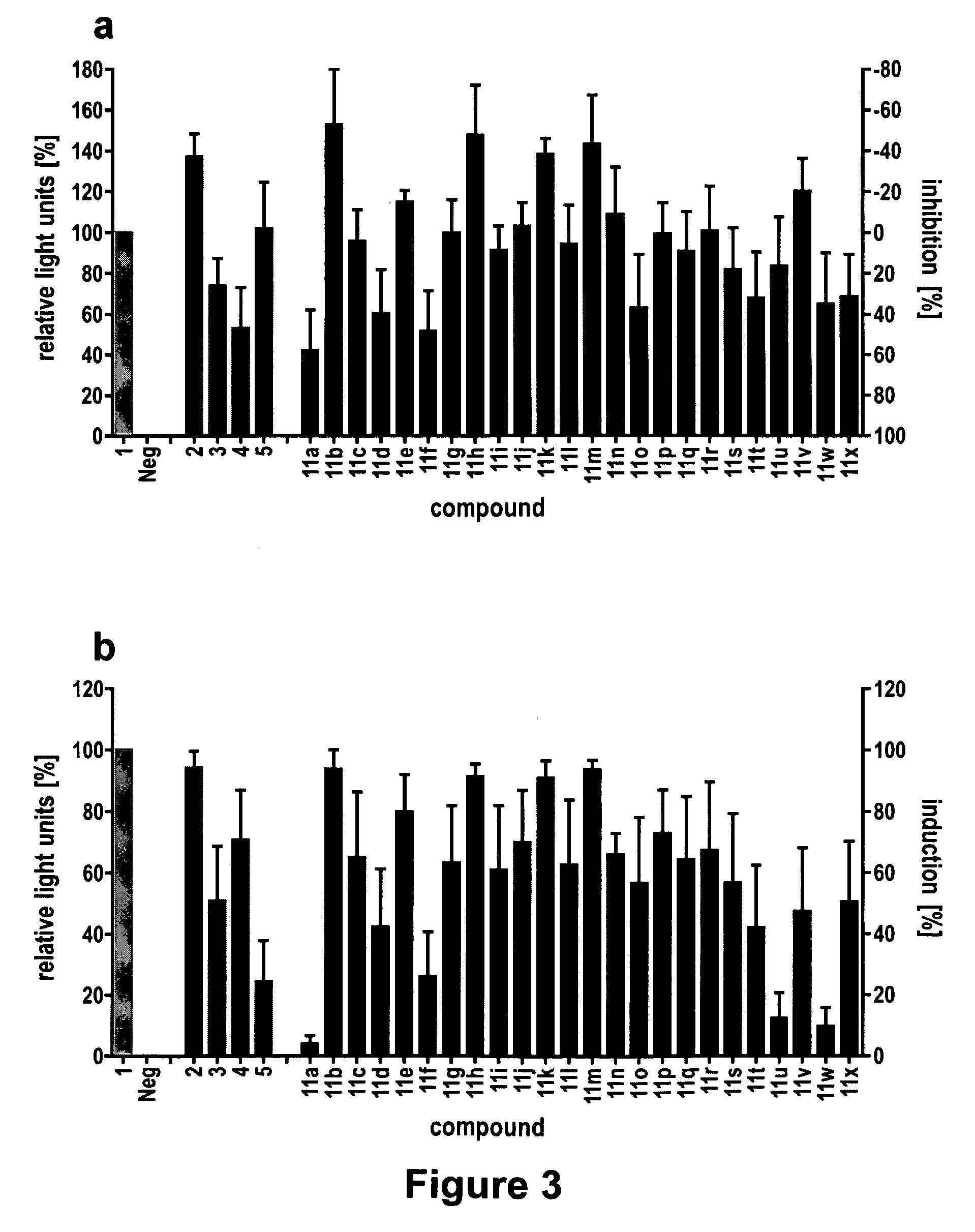
Modulation of Bacterial Quorum Sensing with Synthetic Ligands
a technology of quorum sensing and synthetic ligands, which is applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of inability to selectivise these ligands for different r proteins, scarce potent antagonists, and inability to design new ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
From Antagonist to Super-Agonist
Structural Isomers of N-Phenylacetanoyl-L-Homoserine Lactones Elicit Strong and Opposite Quorum Sensing Responses in Vibrio fischeri
[0169]Abstract: Bacteria monitor their population densities using low molecular weight ligands in a process known as quorum sensing. At sufficient cell densities, bacteria can change their mode of growth and behave as multicellular communities that play critical roles in both beneficial symbioses and in the pathogenesis of infectious disease. The development of non-native ligands that can block quorum-sensing signals has emerged as a promising new strategy to attenuate these divergent outcomes. Here, we report that N-phenylacetanoyl-L-homoserine lactones are capable of either inhibiting or, in some cases, strongly inducing quorum sensing in the bacterial symbiont Vibrio fischeri. Moreover, simple structural modifications to these ligands have remarkable effects on activity. For example, movement of a single substituent o...
example 2
Small Molecule Modulation of Quorum Sensing Revealed by the Systematic Evaluation of Synthetic Ligands Across Three Gram-Negative Bacterial Species
[0206]Abstract: Bacteria monitor their population densities using low molecular weight ligands in a phenomenon called quorum sensing. At high cell densities, bacteria use this chemical signaling process to change their mode of growth and behave as multicellular communities that play essential roles both in the pathogenesis of infectious disease and in beneficial symbioses. There is intense and growing interest in the development of synthetic ligands that can intercept quorum sensing signals and attenuate these divergent outcomes. Both broad spectrum and species specific modulators of quorum sensing hold significant value as small molecule tools for fundamental studies of this complex cell-cell signaling process and for future biocontrol applications. However, synthetic inhibitors or activators of quorum sensing in one species, let alone m...
— example 2
REFERENCES AND NOTES—EXAMPLE 2
[0284]Bassler, B. L.; Losick, R. Cell 2006, 125, 237-246.[0285]Waters, C. M.; Bassler, B. L. Ann. Rev. Cell Dev. Biol. 2005, 21, 319-346.[0286]Fuqua, C.; Parsek, M. R.; Greenberg, E. P. Annu. Rev. Genet. 2001, 35, 439[0287]de Kievit, T. R.; Iglewski, B. H. Infect. Immun. 2000, 68, 4839-4849.[0288]Hall-Stoodley, L.; Costerton, J. W.; Stoodley, P. Nat. Rev. Microbiol. 2004, 2, 95-108.[0289]Winans, S. C. Trends Microbiol. 1998, 6, 382-383.[0290]Greenberg, E. P., Quorum Sensing in Gram-Negative Bacteria: An Important Signaling Mechanism in Symbiosis and Disease. In Microbial Ecology and Infectious Disease, Rosenberg, E., Ed. American Society for Microbiology: Washington, D.C., 1999; pp 112-122.[0291]Ruby, E. G. Annu. Rev. Microbiol. 1996, 50, 591-624.[0292]Lyon, G. J.; Muir, T. W. Chem. Biol. 2003, 10, 1007-1021.[0293]Gonzalez, J. E.; Keshavan, N. D. Microbiol. Mol. Biol. Rev. 2006, 70, 859-875.[0294]Fuqua, C.; Greenberg, E. P. Nat. Rev. Mol. Cell. Biol. 20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com