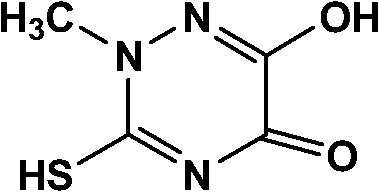
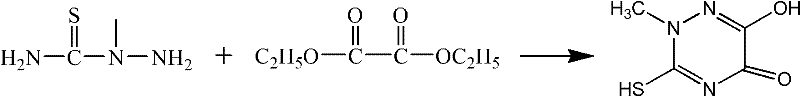
Method for synthesizing thiotriazinone
A synthesis method and technology of triazine ring, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of long overall reaction time, waste of time and raw materials, low reaction yield and the like, so as to shorten the reaction time, reduce the cost of raw materials, and reduce the operation effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Add the mixture of 10.5g of the reaction material 2-methylthiosemicarbazide, 230ml of ethanol, 10.7g of ammonium chloride and 32.1ml of 10wt% hydrochloric acid solution into a three-necked flask, and add diethyl oxalate dropwise when the temperature is controlled at 40°C 14.7 g of ester was added dropwise in 30 minutes, then heated to reflux for 3 hours, cooled to 10°C, filtered and dried to obtain a crude triazine ring.
[0019] (2) Add an appropriate amount of distilled water and the crude product of triazine ring in the reaction flask, heat up, add a small amount of activated carbon after the material is completely dissolved, continue to stir for 30min, heat filtration, cooling, crystallization, filtration, obtain product 12.5g, yield 78.6%.
Embodiment 2
[0021] (1) Add the mixture of 2-methylthiosemicarbazide 10.5g, ethanol 240ml, 11.2g ammonium chloride and 33.6ml 10wt% hydrochloric acid solution into the three-necked flask, and add diethyl oxalate dropwise when the temperature is controlled at 40°C 16.2 g of ester was added dropwise in 30 minutes, then heated to reflux for 3 hours, cooled to 10°C, filtered and dried to obtain a crude triazine ring.
[0022] (2) Add an appropriate amount of distilled water and the crude product of triazine ring in the reaction flask, heat up, add a small amount of activated carbon after the material is completely dissolved, continue to stir for 30min, heat filtration, cooling, crystallization, filtration, obtain product 13.1g, yield 82.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com