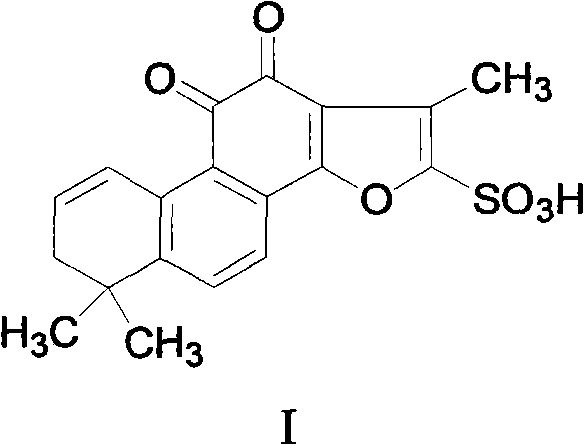
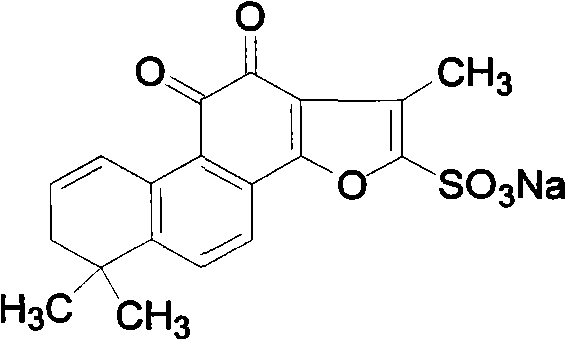
Dehydro-tanshinone IIA sulfoacid and application thereof
A technology of hydrogen salvia miltiorrhiza and tanshinone, which is applied in the field of medicine, can solve the problems of effective monitoring, impurities, and high content, and achieve the effect of ensuring safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 The separation and preparation of dehydrotanshinone IIA sodium sulfonate
[0020] The preparation method of dehydrotanshinone IIA sodium sulfonate:
[0021] a) Take 0.5 g of tanshinone IIA sodium sulfonate raw material, add it to methanol, stir and dissolve, and prepare a sample on a silica gel plate;
[0022] b) Expand the silica gel plate with chloroform / methanol (5:1), there is another dark spot under the dark spot of sodium tanshinone IIA sulfonate, scrape this dark spot and elute with methanol;
[0023] c) Concentrate the eluent, prepare a silica gel plate and load the sample, develop with chloroform / methanol (10:1), scrape the dark spots and elute with methanol, evaporate the eluent to dryness, and repeat the operation many times to obtain the compound dehydrogenation Sodium Tanshinone IIA Sulfonate. 1 H-NMR (CDCl 3 ): δ7.90(d, 1H), 7.75(d, 1H), 7.71(s, 1H), 6.35(s, 1H), 2.47(s, 3H), 2.31(s, 2H), 1.31(s, 6H).
Embodiment 2
[0024] Example 2 The separation and preparation of dehydrotanshinone IIA sulfonic acid
[0025] The preparation method of dehydrotanshinone IIA sulfonic acid:
[0026] a) Take 0.5 g of sodium tanshinone IIA sulfonate raw material, add it to methanol, add a little hydrochloric acid dropwise, stir and dissolve, and prepare a sample on a silica gel plate;
[0027] b) Expand the silica gel plate with chloroform / methanol (8:1), there is another dark spot under the dark spot of the raw material, scrape this dark spot and elute with methanol;
[0028] c) Concentrate the eluent, prepare a silica gel plate and load the sample, develop with chloroform / methanol (15:1), scrape the dark spots and elute with methanol, evaporate the eluent to dryness, and repeat the operation many times to obtain the compound dehydrogenation Tanshinone IIA sulfonic acid.
Embodiment 3
[0029] Embodiment 3 Synthesis of dehydrotanshinone IIA sodium sulfonate
[0030] Take about 100 mg of dehydrotanshinone IIA sulfonic acid, add methanol until just dissolved, then add an appropriate amount of sodium bicarbonate, stir, filter, spin the filtrate, and recrystallize from methanol to obtain the compound shown in formula II.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com