Donepezil transdermal patch
A technology of donepezil and patch, applied in the fields of sheet delivery, neurological diseases, medical preparations of non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of donepezil transdermal patch
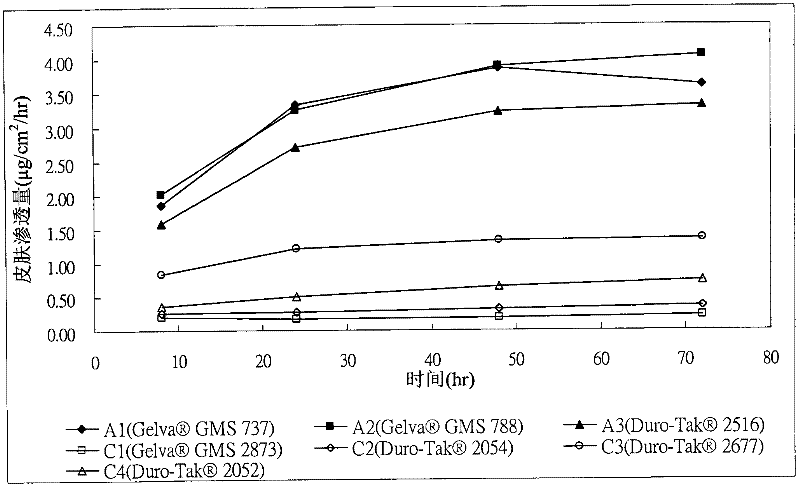
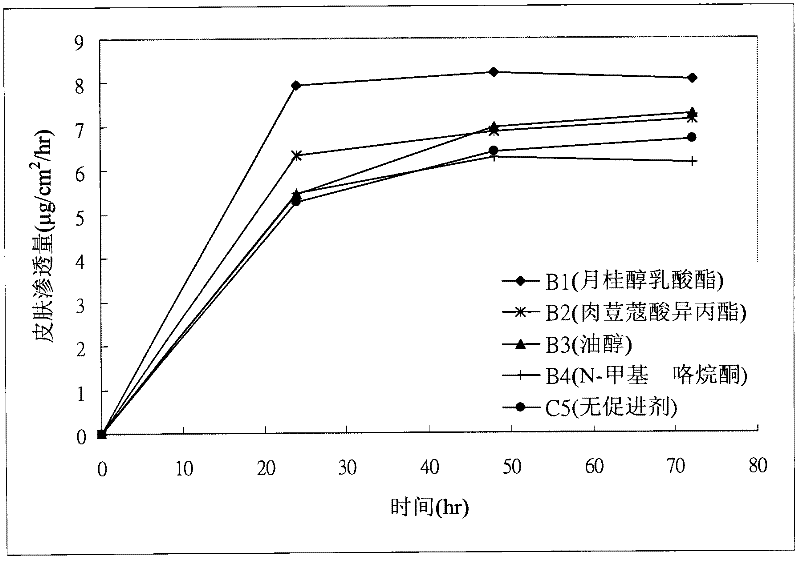
[0042] Donepezil transdermal patches including the ingredients shown in Tables 1 and 2 below in an acrylic pressure-sensitive adhesive matrix layer were manufactured. First, the donepezil free base listed in Table 1 and Table 2, acrylic pressure sensitive adhesive and penetration enhancer (if used), are mixed in ethyl acetate at room temperature as a solution and then formed to a thickness of about 95 microns. The solution is coated on a polyethylene terephthalate (PET) release liner by means of a matrix layer of a pressure-sensitive adhesive, dried to remove ethyl acetate, and then bonded to a polyester support layer Together, to make donepezil transdermal patches, wherein samples A1 to A3 and B1 to B4 are examples of the present invention, and samples C1 to C5 are comparative examples.
[0043] Table 1
[0044]
[0045] q.s.: enough
[0046] Table 2
[0047]
[0048] q.s.: enough
Embodiment 2
[0049] Example 2: Skin Flux Method
[0050] 1. Samples: transdermal donepezil patches for samples A1 to A3 and B1 to B4 and samples C1 to C5
[0051] 2. Test methods and conditions
[0052] (1) Skin penetration device:
[0053] Cut the skin into a predetermined area, such as 1cm 2 The skin slices were placed in side by side cells of the skin permeation device, and the outer jacket of the side by side cells was controlled at 32°C. After the release liner of the donepezil transdermal patch was peeled off, the The stromal layer is adhesively fixed to the skin sheet. 3.5ml 20% PEG 400 was added to the chamber as medium. Take 0.5ml of medium at the appropriate time and test, and then add 0.5ml of medium to the chamber
[0054] (2) Receptor medium: 20% polyethylene glycol 400 (PEG 400)
[0055] (3) Temperature: 32°C
[0056] (4) Sampling time: 24th, 48th and 72nd hours
[0057] (5) Sample analysis: analyze samples using chromatographic techniques
[0058] 3. Results
[005...
Embodiment 3
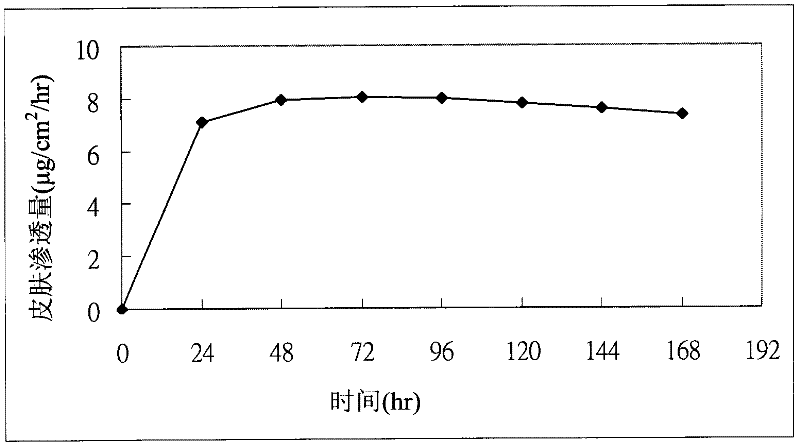
[0066] Embodiment 3: Human body preliminary test result
[0067] Take the same size (about 20mg / 20cm 2 ) of sample B1 in Table 2, were respectively pasted on the chest of 4 subjects for administration; and donepezil of 5 mg / tablet was orally administered to the other 4 subjects. Blood was drawn at different time points to measure the concentration of donepezil in the plasma of the subjects, so as to determine the skin penetration rate of donepezil. Figure 4 It shows the results of the human body preliminary test of the donepezil transdermal patch of the present invention and the human body oral test results of donepezil. like Figure 4 As shown, use donepezil transdermal patch (20mg / 20cm) of the present invention 2 ) subjects had a similar maximum concentration of donepezil in plasma as subjects taking oral donepezil lozenges (5 mg / tablet).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com