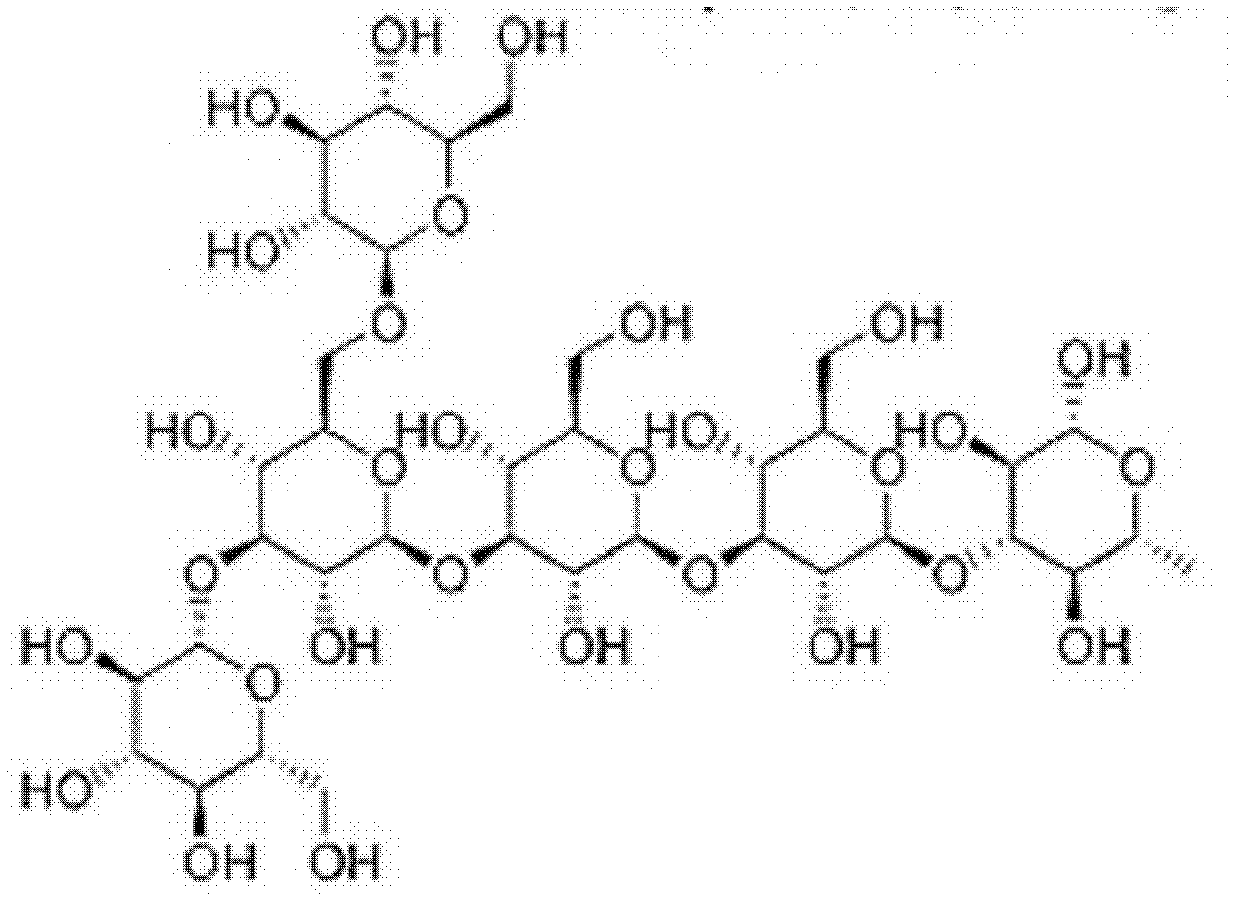
Application of beta-dextran in preparation of human dendritic cell tumor vaccine
A dendritic cell and tumor vaccine technology, which is applied in the application field of beta-glucan in the preparation of human dendritic cell tumor vaccine, can solve the problems such as undiscovered human dendritic cell tumor vaccine, and achieves the improvement of immunity. Potency, the effect of promoting proliferative ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0021] The application of the β-glucan of this example in the preparation of human dendritic cell tumor vaccine has the following two steps:
[0022] ① Obtaining immature human dendritic cells:
[0023] Use the Fresenius blood cell separator and its matching disposable consumption tubing, select the double-needle mononuclear cell program, and input the corresponding parameters of the person to be collected (sex, height, weight, hematocrit, etc.), according to the collected person Adjust the blood flow rate to 50mL / min to 80mL / min according to the specific situation, and the ratio of anticoagulant to whole blood is 1:10. Mononuclear cells are separated from human peripheral blood, and the volume of processed whole blood does not exceed twice the volume of own blood. , to obtain peripheral blood mononuclear cells.
[0024] Place the peripheral blood mononuclear cells obtained above into a 15 mL centrifuge tube, take 10 μL of cells for counting, and then add anti-human CD14 + I...
experiment example 1
[0030] (Experimental example 1, immature human dendritic cells can phagocytose β-glucan)
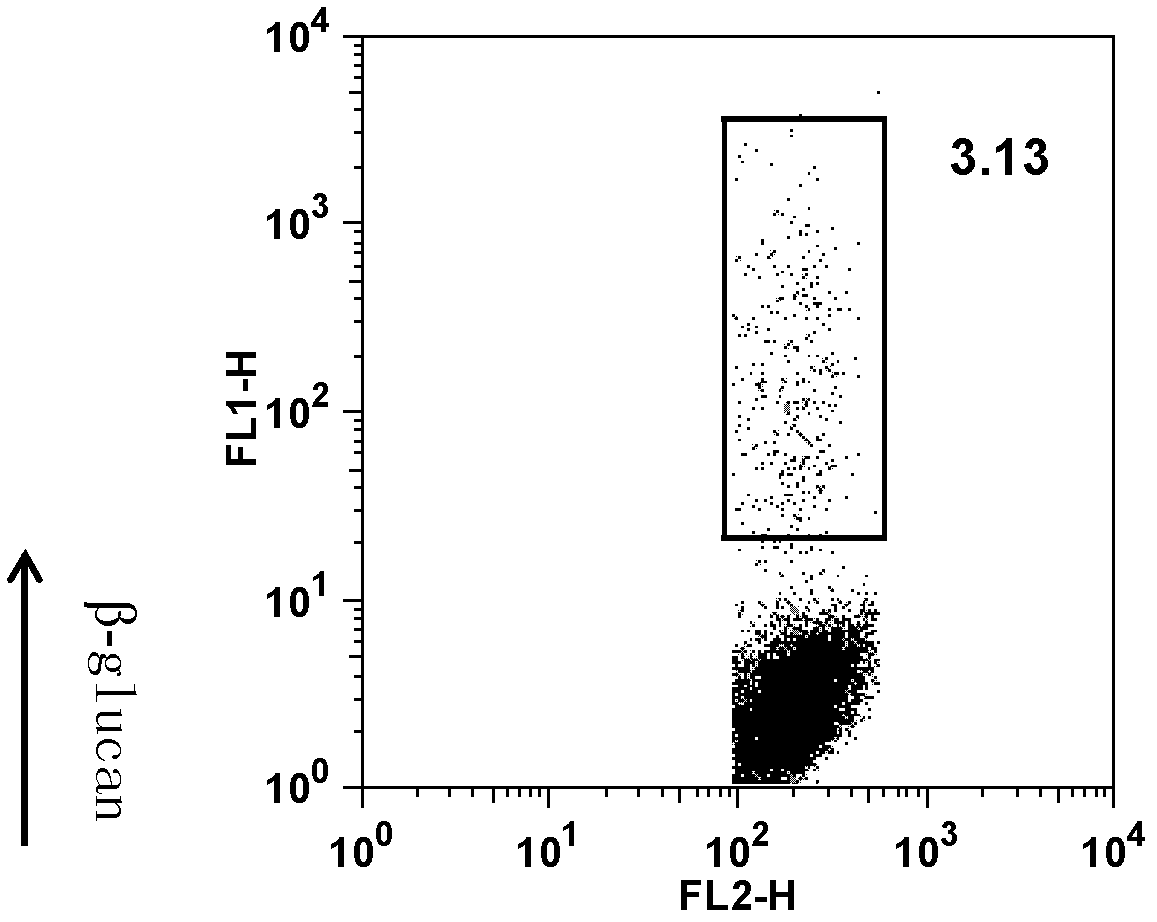
[0031] Take 5×10 5 Step 1 of Example 1 ①The immature human dendritic cells obtained by culturing for 120 h and fluorescein DTAF-labeled β-glucan (concentration: 20 μg / mL) were cultured at 37°C for 1 h, immediately placed on ice, and washed with pre- Wash with cold PBS 3 times and perform flow cytometry. See the experimental results figure 2 .
[0032] Depend on figure 2 It can be seen that immature human dendritic cells can phagocytose β-glucan.
experiment example 2
[0033] (Experimental example 2, the effect of β-glucan on the expression of co-stimulatory molecules and the concentration of cytokines)
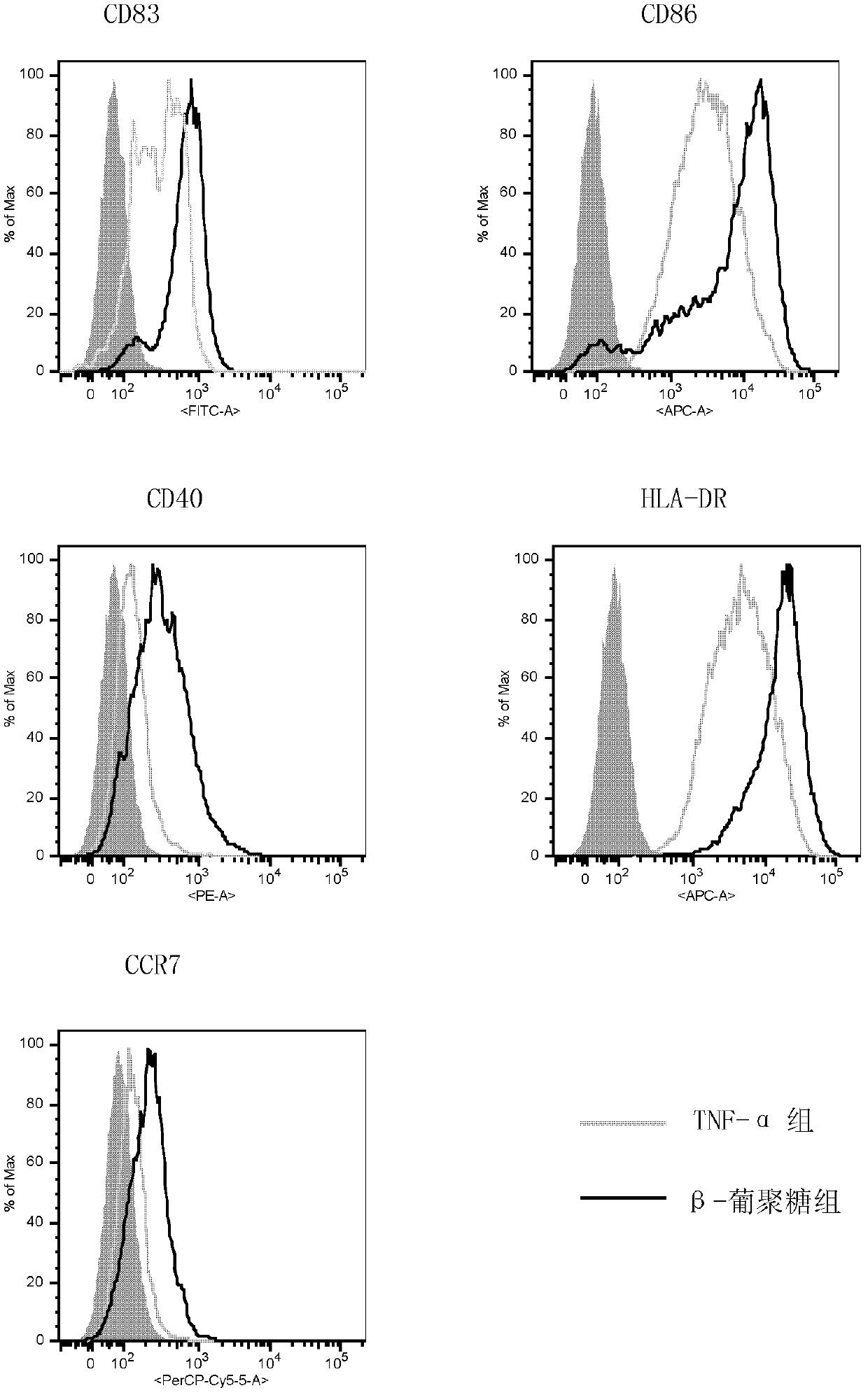
[0034] In this experimental example, three groups of human dendritic cells were used to detect CD83, CD86, CD40, HLA-DR, and CCR7 surface markers to determine maturity. The first group is the immature human dendritic cells collected by centrifugation in step ① of Example 1 and cultured for 120 hours. The second group was human dendritic cells 48 hours after the immature human dendritic cells obtained in the first group were stimulated by β-glucan (concentration: 100 μg / ml). The third group is the human dendritic cells obtained after stimulating the immature human dendritic cells obtained in the first group by TNF-α (concentration: 20ng / mL) for 48 hours. The experimental results are shown in image 3 .
[0035] Depend on image 3 It can be seen that the expression of co-stimulatory molecules in the second group significantly increased aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com