Application of triarylimidazole compound serving as catalyst in electroorganic synthesis
A triaryl imidazole and organic synthesis technology, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, electrolytic organic production, etc., can solve problems that have not been reported in domestic and foreign literature, and achieve remarkable results Scientific significance and application prospect, high yield, easy preparation and separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
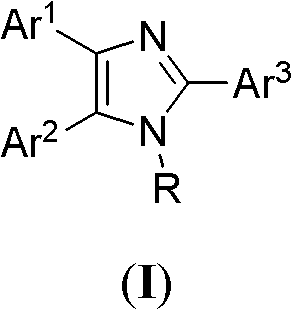
[0017] Example 1: Preparation of 4-methoxybenzaldehyde by catalytic oxidation of 2-(4-bromophenyl)-1-methyl-4,5-diphenyl-1H-imidazole
[0018] Step 1: Synthesis of catalyst 2-(4-bromophenyl)-1-methyl-4,5-diphenyl-1H-imidazole
[0019] Equivalent 1,2-diphenylethanedione, p-bromobenzaldehyde, methylamine, ammonium acetate and 0.015 equivalent of KH 2 PO 4 Mix, heat to 150°C, keep the temperature for about 1.5 hours, then cool to room temperature, and recrystallize the solid with acetone and water to obtain 2-(4-bromophenyl)-1-methyl-4,5-diphenyl- 1H-imidazole, yield: 92%.
[0020]
[0021] Yellow needle; 1 HNMR (400MHz, CDCl 3 ): δ=3.50(s, 3H), 7.15(t, J=7.2Hz, 1H), 7.21(t, J=7.2Hz, 2H), 7.39-7.41(m, 2H), 7.45-7.48(m, 3H), 7.52-7.54(m, 2H), 7.62-7.66(m, 4H).
[0022] Step 2: Catalytic oxidation of 4-methoxybenzyl alcohol by 2-(4-bromophenyl)-1-methyl-4,5-diphenyl-1H-imidazole
[0023] In a 50 mL double-chamber electrolytic cell, dissolve 0.1 mmol of 2-(4-bromophenyl)-1...
Embodiment 2
[0026] Example 2: Preparation of 3-methoxybenzaldehyde by catalytic oxidation of 2-(4-bromophenyl)-1-methyl-4,5-diphenyl-1H-imidazole Step 2 by adding 3-methoxybenzyl Alcohol, other steps are the same as in Example 1 to generate 3-methoxybenzaldehyde, yield: 65%.
[0027]
[0028] 1 HNMR (400MHz, CDCl 3 ): δ=3.88(s, 3H), 7.18-7.19(m, 1H), 7.40(s, 1H), 7.48(d, J=6.4Hz, 2H), 9.98(s, 1H).
Embodiment 3
[0029] Example 3: Preparation of 4-methoxybenzaldehyde by catalytic oxidation of 2-(4-bromophenyl)-1-methyl-4,5-diphenyl-1H-imidazole
[0030] Add 4-methylanisole in step 2, and other steps are the same as in Example 1 to generate 4-methoxybenzaldehyde with a yield of 57%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com