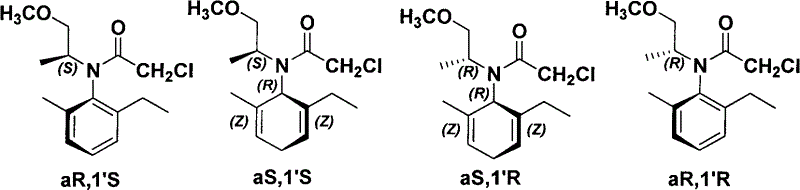
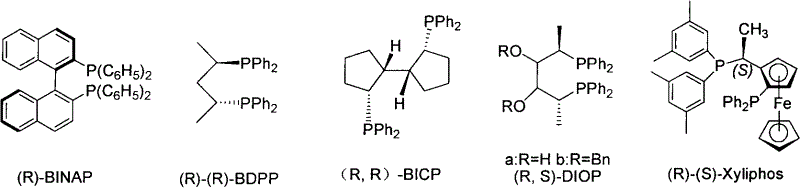
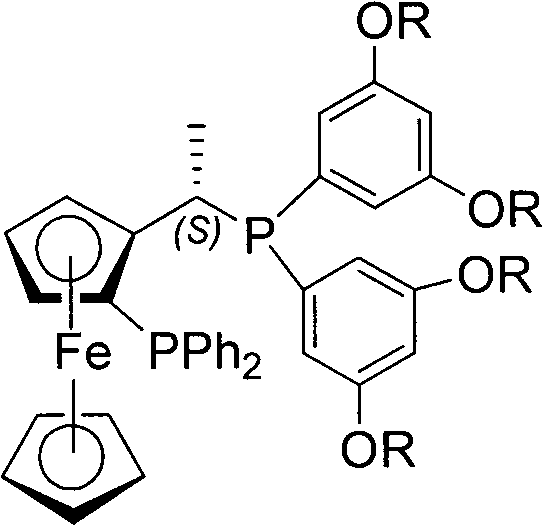
Chiral diphosphine ligand-iridium composite nanocatalyst and application thereof to asymmetric hydrogenation synthesis of (S)-metolachlor
A technology of chiral bisphosphine ligands and nano-catalysts, applied in organic compound/hydride/coordination complex catalysts, preparation of organic compounds, physical/chemical process catalysts, etc. advanced questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 The preparation of ligand Ia (general structural formula I, R=methyl)-iridium composite nanocatalyst IIat
[0018] The chiral bisphosphine ligand Ia (general structural formula I, R=methyl)-iridium composite catalyst is dissolved in ether, added with 20% nano-titanium dioxide to stir, and the ether is removed by rotary evaporation to obtain the ligand Ia-iridium composite nanocatalyst IIat .
Embodiment 2
[0019] Example 2 Preparation of Ligand Ib (such as general structural formula I, R = tert-butyl)-iridium composite nanocatalyst IIbs
[0020] The chiral bisphosphine ligand Ib (such as structural formula I, R = tert-butyl)-iridium composite catalyst is dissolved in ethyl acetate, added with 20% nano-silica and stirred, and the ethyl acetate is removed by rotary evaporation to obtain the compound Bulk Ib-iridium composite nanocatalyst IIbs.
Embodiment 3
[0021] Example 3 Preparation of Ligand Ic (such as general structural formula I, R=ethyl)-iridium composite nanocatalyst IIct
[0022] The chiral bisphosphine ligand Ic (such as structural formula I, R=ethyl)-iridium composite catalyst is dissolved in chloroform, added 20% nano-titanium dioxide to stir, and the solvent is removed by rotary evaporation to obtain the ligand Ic-iridium composite nano-catalyst IIct.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com