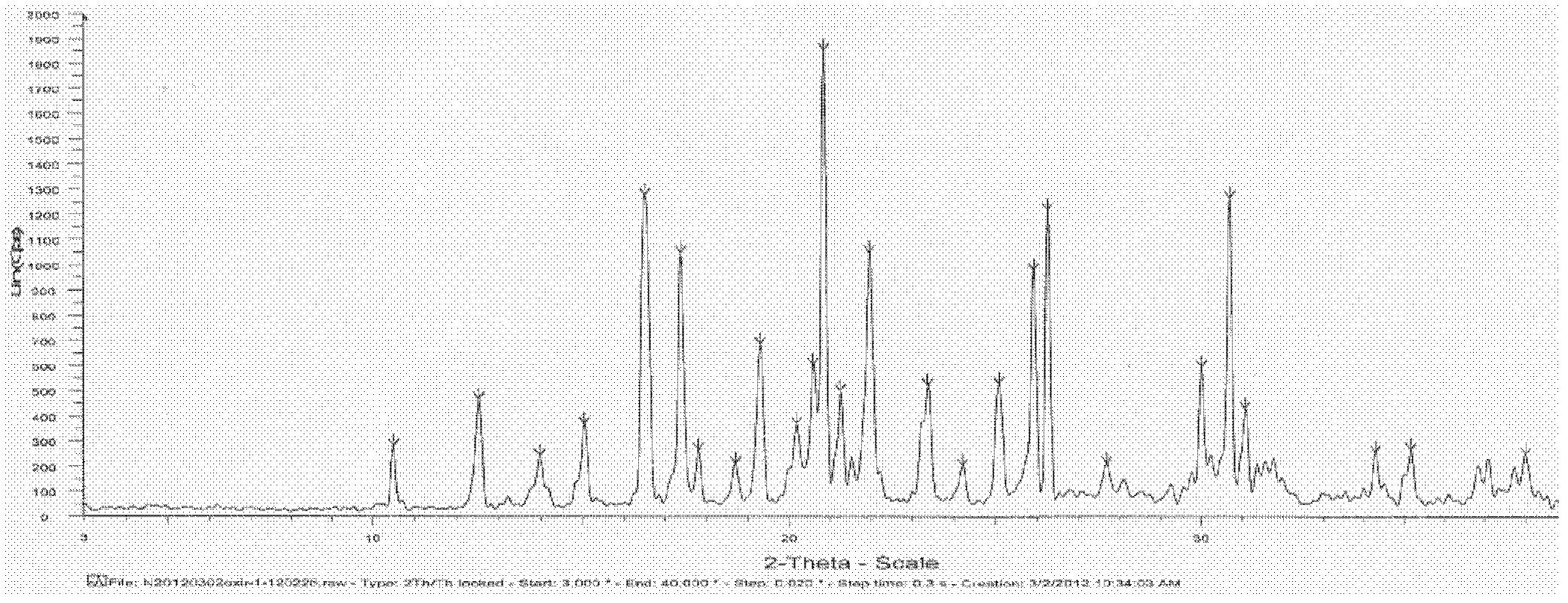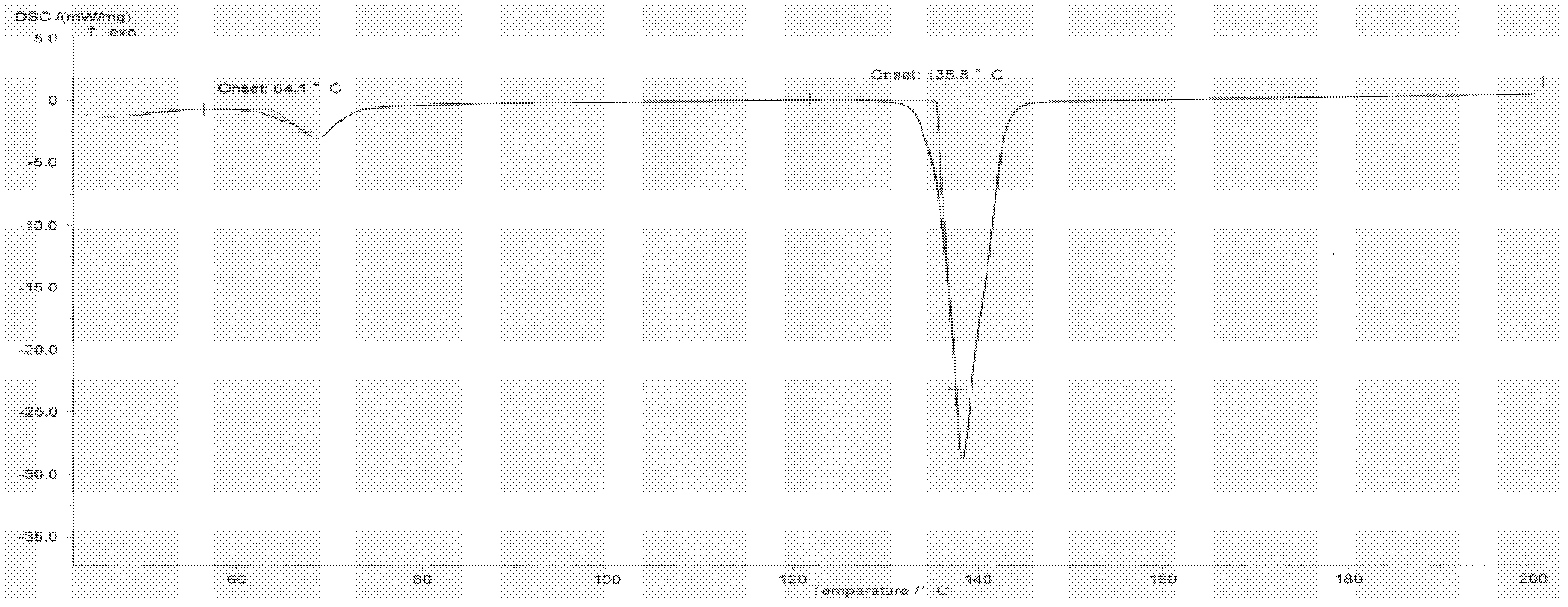Novel crystal formations of levogyration oxiracetam and preparation method thereof
A technology of crystal form and crystallization temperature, applied in the field of new crystal form of levoxiracetam, preparation method and pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
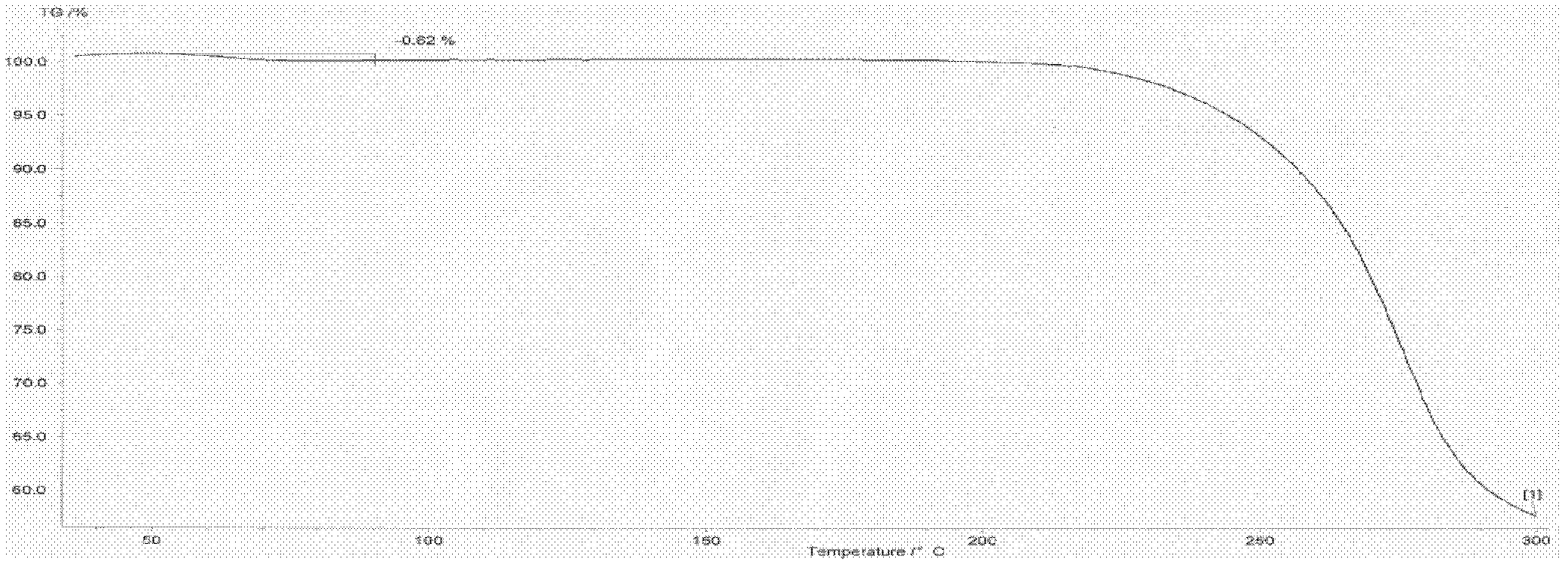
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of Levoxiracetam
[0032] According to the method disclosed in TETRAHEDRON: ASYMMETRY 1992, 3(11), 1431-40, 503.0 g of levoxiracetam was prepared, and the HPLC purity was 99.1%.
[0033] Example 2: Preparation of L-Oxiracetam crystal form A
[0034] 10.0 g of S-oxiracetam was prepared from Example 1, heated with methanol to dissolve it completely, and concentrated to dryness under reduced pressure to obtain crystal form A of levoxiracetam.
[0035] Example 3 Preparation of L-Oxiracetam crystal form A
[0036] take Example 1 10.0 g of Levoxiracetam was prepared, which was completely dissolved by heating with isopropanol, and concentrated to dryness under reduced pressure to obtain crystal form A of Levoxiracetam.
[0037] Example 4 Preparation of L-Oxiracetam crystal form A
[0038] take Example 1 15.0 g of Levoxiracetam was obtained, which was completely dissolved by heating with DMF, and concentrated to dryness under reduced pressure to obtain crystal f...
Embodiment 7
[0043] Example 7 Preparation of L-Oxiracetam crystal form B
[0044] take Example 1 Prepare 50.0g of L-Oxiracetam, dissolve it by heating with a mixed solvent of n-butanol and water (volume ratio 15:1), then cool to 20℃, stir to crystallize, filter with suction, and finally vacuum at 45~55℃ After drying, 40.5 g of L-Oxiracetam crystal form B was obtained with a yield of 81.0%.
Embodiment 8
[0045] Example 8 Preparation of L-Oxiracetam crystal form B
[0046] take Example 1 Prepare 50.0 g of levoxiracetam, dissolve it by heating with a mixed solvent of DMF and water (10:1 by volume), then cool to 35°C, stir to crystallize, filter with suction, and finally vacuum dry at 45-55°C. 36.2g of L-Oxiracetam crystal form B was obtained with a yield of 72.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com