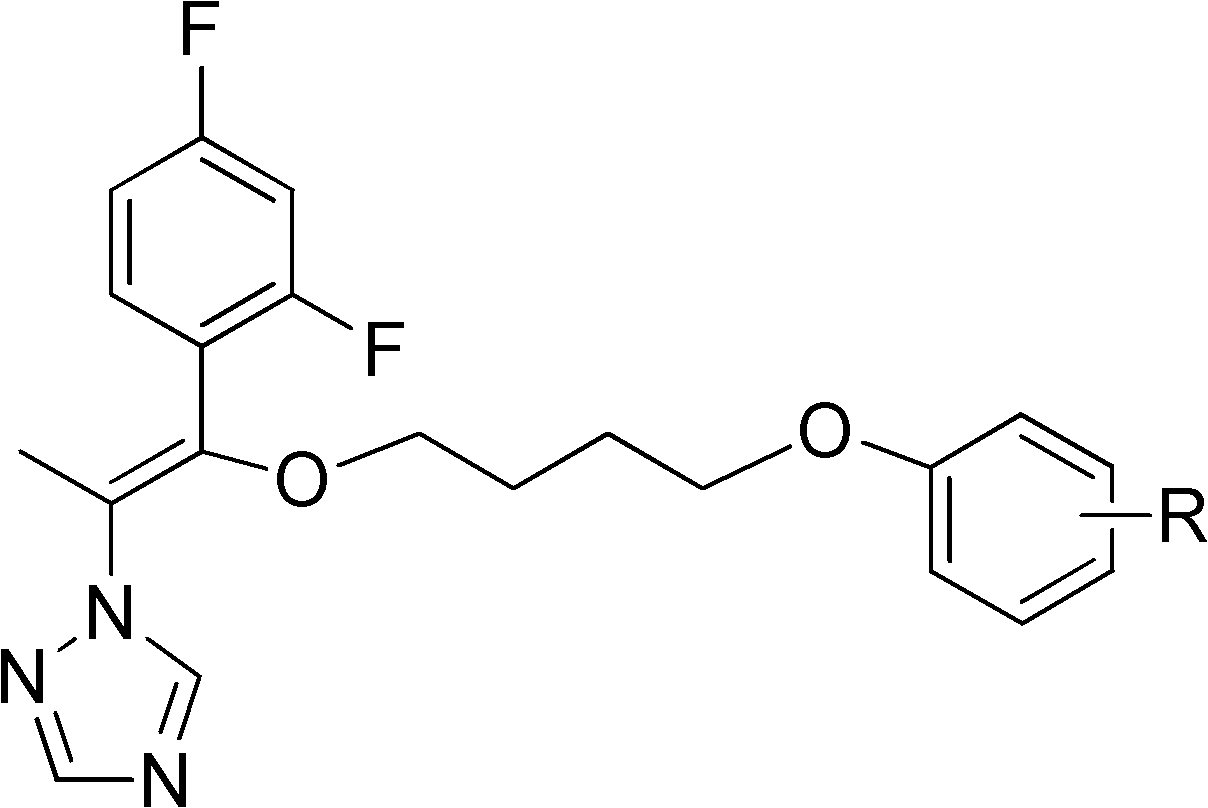
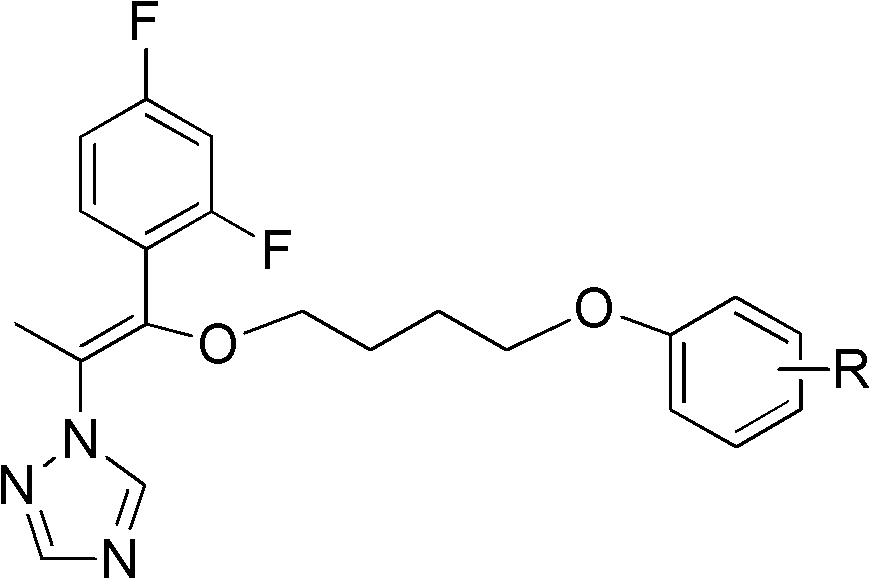
A kind of triazole compound containing alkenyl ether structure and its preparation method and application
A technology of triazoles and compounds, which is applied in the field of medicine, can solve the problems such as the antifungal activity of triazoles compounds that have not been seen, and achieve the effects of environmental friendliness, simple process and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
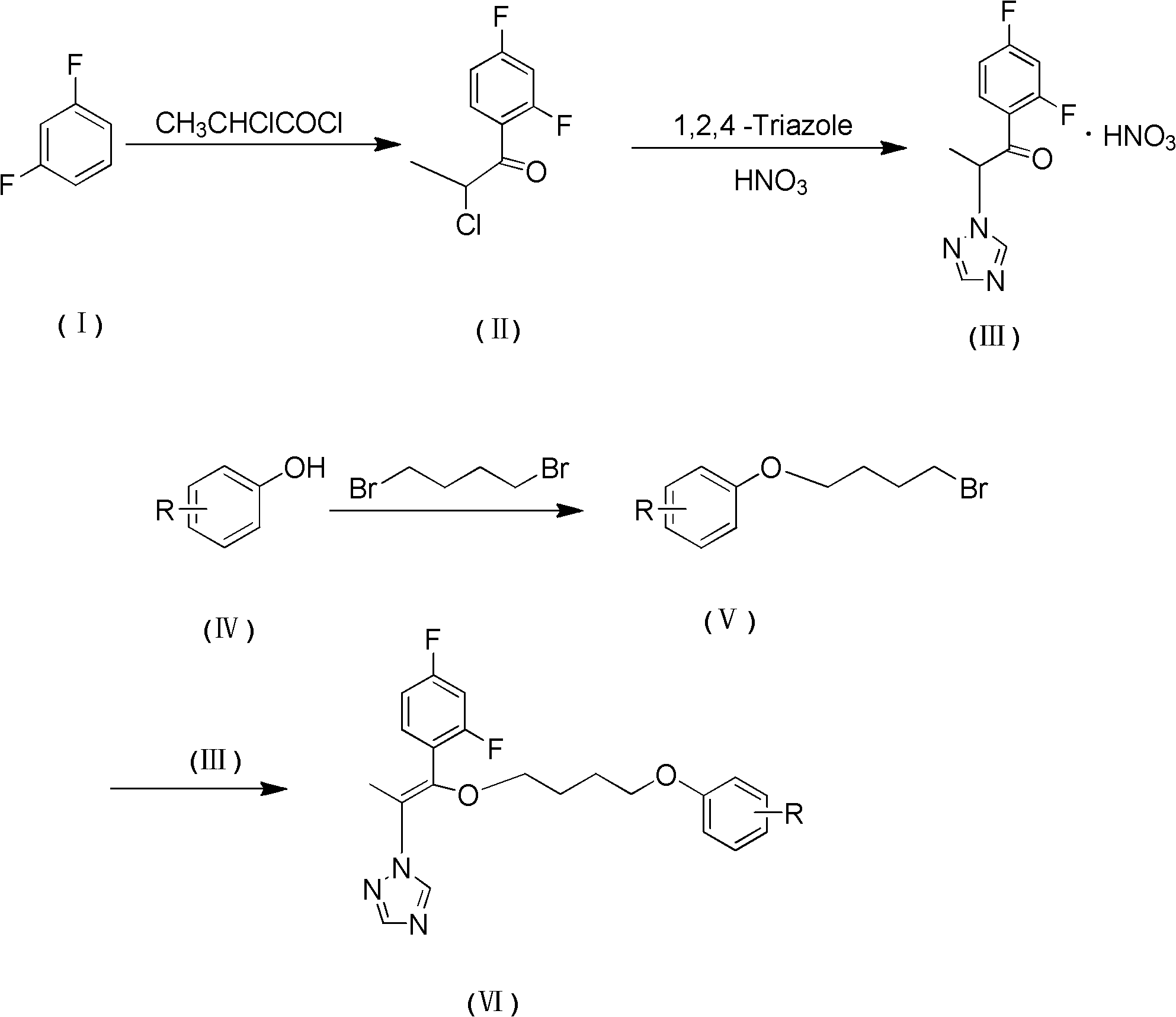
[0033] The synthetic method of compound 1, comprises the steps:
[0034] (1) Preparation of α-chloro-2,4-difluoropropiophenone (II)
[0035] Put 80g (0.6mol) of anhydrous aluminum trichloride and 60ml (0.6mol) of m-difluorobenzene into a 500ml three-neck flask, stir in a water bath at 10°C, add 60ml (0.6mol) of 2-chloropropionyl chloride dropwise, and add dropwise After completion, the temperature was raised to 40 ° C, and the reaction solution was poured into ice water after maintaining this temperature for 6 h. The aqueous phase was extracted three times with 600 ml of dichloromethane, and the organic phases were combined and washed with 5% NaHCO 3 Wash the organic phase twice with 800ml of aqueous solution, then wash with water to pH 7, dry over anhydrous sodium sulfate, filter to obtain a transparent light yellow liquid, and distill under reduced pressure to obtain 88.5g of light yellow oil, which is the compound α-chloro-2,4 -Difluoropropiophenone, yield 72.1%.
[0036]...
Embodiment 2
[0045] The synthetic method of compound 2, comprises the steps:
[0046] (1) Preparation of α-chloro-2,4-difluoropropiophenone (II)
[0047] Put 147g (1.1mol) of anhydrous aluminum trichloride and 60ml (0.6mol) of m-difluorobenzene into a 500ml three-necked flask, stir in a water bath at 13°C, add 90ml (0.9mol) of 2-chloropropionyl chloride dropwise, and add dropwise After completion, the temperature was raised to 50 ° C, and the reaction solution was poured into ice water after maintaining this temperature for 5 h. The aqueous phase was extracted three times with 600 ml of dichloromethane, and the organic phases were combined and washed with 5% NaHCO 3 Wash the organic phase twice with 800ml of aqueous solution, then wash with water to pH 7, dry over anhydrous sodium sulfate, filter to obtain a transparent light yellow liquid, and distill under reduced pressure to obtain 118.9g of light yellow oil, which is the compound α-chloro-2,4 -Difluoropropiophenone, yield 96.8%.
[0...
Embodiment 3
[0057] The synthetic method of compound 3, comprises the steps:
[0058] (1) Preparation of α-chloro-2,4-difluoropropiophenone (II)
[0059] 253g (1.9mol) of anhydrous aluminum trichloride and 60ml (0.6mol) of m-difluorobenzene were placed in a 500ml three-necked flask, stirred in a water bath at 17°C, 120ml (1.2mol) of 2-chloropropionyl chloride was added dropwise, and After completion, the temperature was raised to 60 ° C, and the reaction solution was poured into ice water after maintaining this temperature for 3.5 h. The aqueous phase was extracted three times with 750 ml of dichloromethane, and the organic phases were combined and washed with 5% NaHCO 3 Wash the organic phase twice with 800ml of aqueous solution, then wash with water to pH 7, dry over anhydrous sodium sulfate, filter to obtain a transparent light yellow liquid, and distill under reduced pressure to obtain 108.4g of light yellow oil, which is the compound α-chloro-2,4 -Difluoropropiophenone, yield 88.3%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com