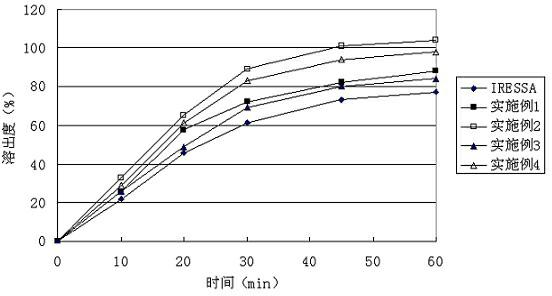
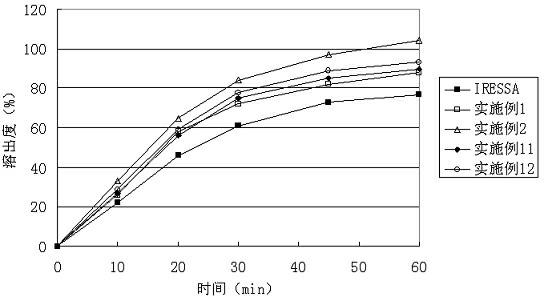
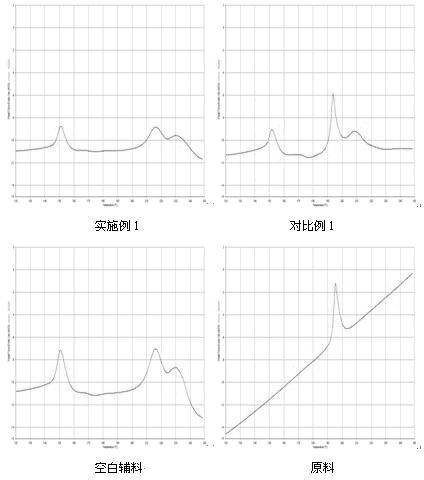
Gefinitib medicinal composite and method for preparing same
A gefitinib and composition technology, which is applied in the field of gefitinib-containing pharmaceutical compositions and the preparation thereof, can solve the problems of low bioavailability, complicated preparation process, low gefitinib bioavailability and the like , to achieve the effect of excellent quality, simple and easy preparation process, and good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] components Weight (g) content(%) Gefitinib 50.0 50.0 lactose monohydrate 32.7 32.7 microcrystalline cellulose 10.0 10.0 Croscarmellose Sodium 4.0 4.0 Povidone 2.0 2.0 Sodium dodecyl sulfate 0.3 0.3 Magnesium stearate 1.0 1.0 Total 100.0 100.0
[0046] Preparation Process:
[0047] i) povidone and sodium lauryl sulfate are dissolved in water to form a granulation solution;
[0048] ii) Weigh uncrushed gefitinib, lactose, microcrystalline cellulose, and croscarmellose sodium powder according to the prescription amount and mix them evenly;
[0049] iii) adding granulation solution to granulate;
[0050] iv) drying the resulting granules using a fluidized bed;
[0051] v) Whole grains, add magnesium stearate, and mix well;
[0052] vi) Compressed into tablets.
Embodiment 2
[0054] Prescription is identical with embodiment 1.
[0055] Preparation Process:
[0056] i) Gefitinib, povidone and sodium lauryl sulfate are dissolved in aqueous hydrochloric acid solution at pH=1 to form a granulation solution;
[0057] ii) Weigh lactose, microcrystalline cellulose, and croscarmellose sodium powder according to the prescription amount and mix them evenly;
[0058] iii) adding granulation solution to granulate;
[0059] iv) drying the resulting granules using a fluidized bed;
[0060] v) Whole grains, add magnesium stearate, and mix well;
[0061] vi) Compressed into tablets.
Embodiment 3
[0063] components Weight (g) content(%) Gefitinib 61.5 61.5 microcrystalline cellulose 25.0 25.0 Croscarmellose Sodium 7.5 7.5 Povidone 3.5 3.5 Sodium dodecyl sulfate 0.5 0.5 Magnesium stearate 2 2 Total 100.0 100.0
[0064] Preparation Process:
[0065] i) povidone and sodium lauryl sulfate are dissolved in water to form a granulation solution;
[0066] ii) Weigh uncrushed gefitinib, microcrystalline cellulose and croscarmellose sodium powder according to the prescription amount and add them to the fluidized bed, and top-spray granulation with granulation solution;
[0067] iii) Whole grains, add magnesium stearate, and mix well;
[0068] iv) Compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com