Insecticidal protein Cryl A. 301, expression vector and application thereof
A cry1a.301, insect-resistant protein technology, applied in the field of genetic engineering and biological control, can solve the problems of lack of insect-resistant varieties, ecological imbalance, environmental pollution, etc., to expand the insect-resistant spectrum, reduce the amount of use, and reduce environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of Cry1A.301 gene
[0032] Through codon optimization, Cry1Ab and Cry1F were synthesized (the synthesis work was completed by Sangon Bioengineering (Shanghai) Co., Ltd.) and then the first and second functional domains of Cry1Ab and the first functional domain of Cry1F were synthesized by the SOE method (1992, Cai Yuyang). The three functional domains are spliced together. It is generally believed that the Bt protein is composed of three domains. Domain I includes 250-300 amino acids at the N-terminal of the active insecticidal protein and consists of 7 α-helices, one of which is Strong hydrophobicity, located in the center of domain I, surrounded by the other six α-helices. It has been proven that it is related to the toxicity of insecticidal proteins. The hydrophobic α-helix has the ability to insert into the midgut cell membrane of sensitive insects and form pores; domain II consists of three anti-parallel β sheets to form a triangular shape st...
Embodiment 2
[0033] Example 2 Expression of Cry1A.301 gene in prokaryotic system and detection of product toxicity
[0034] In order to detect the in vitro expression of the modified Cry1A.301 gene and its toxicity to the corn borer, we constructed a Bt prokaryotic expression vector. According to the needs of cloning the Bt gene, the NdeI endonuclease recognition site sequence AAGGAGATATACATA was added to the 5' end of the primer sequence, and the XhoI endonuclease recognition site sequence GGTGGTGGTGCTCGAG was added to the 3' end. The designed primer sequence is: F: AAGGAGATATACATA TGGACAACAACCCGAAC,R: GGTGGTGGTGCTCGAG CTCGAGTGTGGCAGTAAC. Using the spliced Cry1A.301 DNA as a template, the Cry1A.301 gene was amplified with adapter-added primers, and the Cry1A.301 gene fragment was recovered and purified with a gel extraction kit. Digest pET30a with restriction endonucleases NdeI and XhoI, recover and purify. The two fragments were ligated, and the resulting prokaryotic expression p...
Embodiment 3
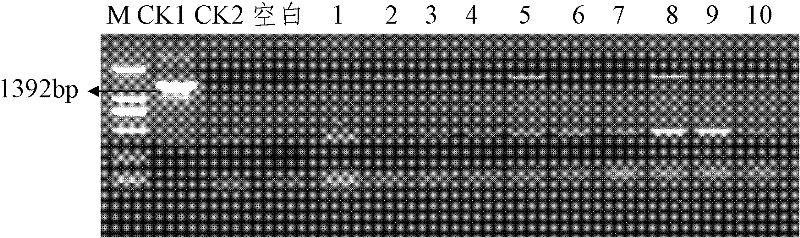
[0046] Example 3 Expression of Cry1A.301 Gene in Transgenic Plants and Toxicity Detection of Expression Products
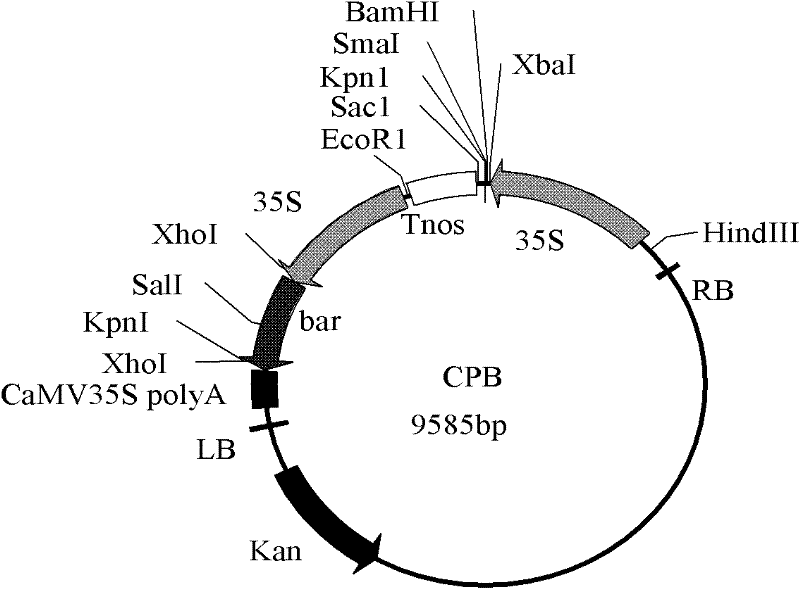
[0047] Add the SmaI endonuclease recognition site sequence CTCTAGAGGATCCCC and TCGAGCTCGGTACCC at the 5' and 3' ends of the primer sequence respectively, and design the primer as: F: 5' CTCTAGAGGATCCCC ATGGACAACAACCCGAAC3'; R: 5' TCGAGCTCGGTACCC CTACTCGAGTGTGGCAGTAAC 3', using the synthesized Cry1A.301 nucleotide sequence as a template, using primers with adapters to amplify, amplification program: 94°C pre-denaturation for 5 minutes, 94°C denaturation for 30s, 55°C annealing for 30s, 68°C extension for 2min, 35 cycles, extended at 68°C for 10 min, and the target fragment was recovered with a gel recovery purification kit. At the same time, the plant expression vector CPB was digested with SmaI, and the CPB fragment after digestion was recovered and purified. The two fragments were ligated (In-Fusion HD cloning kit, Clontech) to construct the eukaryotic expres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com