Helianthus tuberosus fructan-exohydrolases (Ht-FEH) gene and application thereof
A kind of fructan, hydrolase technology, applied in the field of genetic engineering, can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Cloning of Example 1 Ht1-FEH Gene
[0043] The young leaves of the Jerusalem artichoke variety "Nanyu No. 1" at the 4th leaf stage were taken, and the total plant RNA was extracted according to the instructions of the OMEGA plant RNA extraction kit, and the cDNA template was obtained by two-step reverse transcription with the TaKaRa reverse transcription kit.
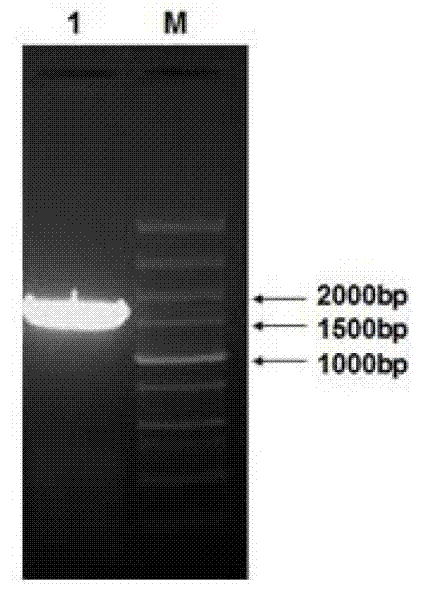
[0044] Blast the existing 1-FEH gene coding sequence (coding sequence, CDS) sequence of other species into Jerusalem artichoke (NCBI) and sunflower EST database (http: / / compbio.dfci.harvard.edu / tgi / plant.html), The EST sequence with high similarity was screened and spliced with DNAstar software to obtain an EST sequence (TC4077) with a full length of 1792 bp. The EST sequence was compared with the existing 1-FEH gene CDS sequence and found that the EST sequence was similar to that of chicory. The CDS sequence similarity of Ci1-FEH I (AJ242538) is 78.3%, and there are start codon ATG and stop codon TGA. Therefo...
Embodiment 2
[0061] According to the cloned Ht 1-FEH CDS sequence, the semi-quantitative expression primer qFEH was designed with Primer Premier 5.0, and the expression pattern of Ht 1-FEH in Jerusalem artichoke was analyzed using the 18S ribosome-encoded gene as an internal reference. The PCR reaction system and procedure were the same as in Example 1, and the number of cycles was 25. The primer sequences are as follows:
[0062] qFEH-F:5'-TGCGTCAAAGTCATTCTA-3' (SEQ ID NO.5)
[0063] qFEH-R:5'-CCATAACTCCCACTCGTA-3' (SEQ ID NO.6)
[0064] 18S-F:5'-TACCGTCCTAGTCTCAACCA-3' (SEQ ID NO.7)
[0065] 18S-R:5'-AACATCTAAGGGCATCACAG-3' (SEQ ID NO.8)
[0066] The transcription level of Ht 1-FEH in different organs and tuber development and germination of Jerusalem artichoke was detected by semi-quantitative RT-PCR ( image 3 ). The expression of Ht 1-FEH was the highest in germinated tubers, and the expression of Ht 1-FEH increased with time after germination; Ht 1-FEH was expressed in mature le...
Embodiment 3
[0067] The construction of embodiment 3 expression vector
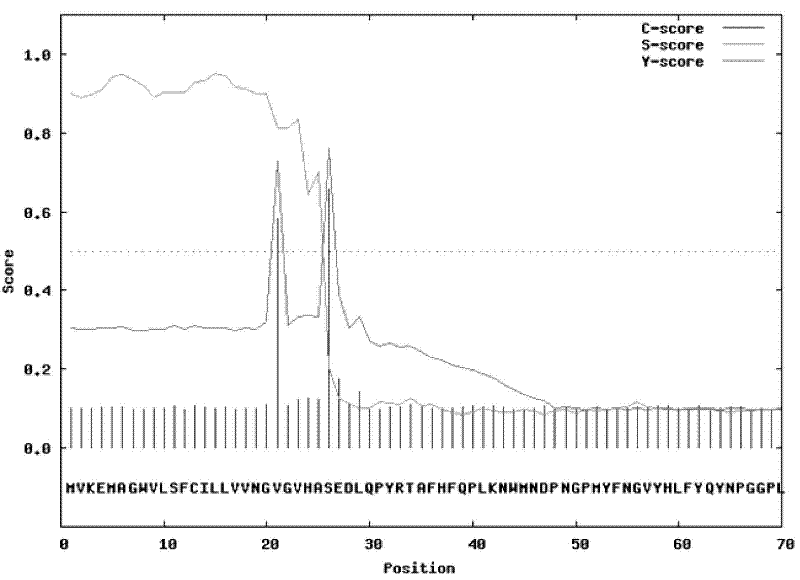
[0068] According to the sequence analysis results of the fructan exohydrolase gene Ht 1-FEH, the upstream primer removed the predicted N-terminal signal peptide sequence: MVKEMAGWVLSFCILLVVNGVGVHA, a total of 25 amino acids, and the upstream primer was designed from the 26th amino acid, plus Xho I Restriction site: C|TCGAG, plus Kex2 signal cleavage sequence: AAGAGA, plus protective base: CCG, use the yeast α factor leader peptide sequence in the vector as the signal peptide to construct a secretory expression vector to The recombinant fructan exohydrolase can be secreted outside the cells of Pichia pastoris. The downstream primer removes the stop codon of the sequence itself, and adds a fusion 6×His tag after the expression frame to make the recombinant protein a fusion protein, which is convenient for purification and detection after protein expression, plus an Xba I restriction site: T|CTAGA , plus the protective ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com