Novel lloperidone pharmaceutical co-crystal and preparation method thereof
A technology of iloperidone and drugs, applied in the field of new iloperidone drug co-crystal and its preparation, to achieve the effect of improving bioavailability and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
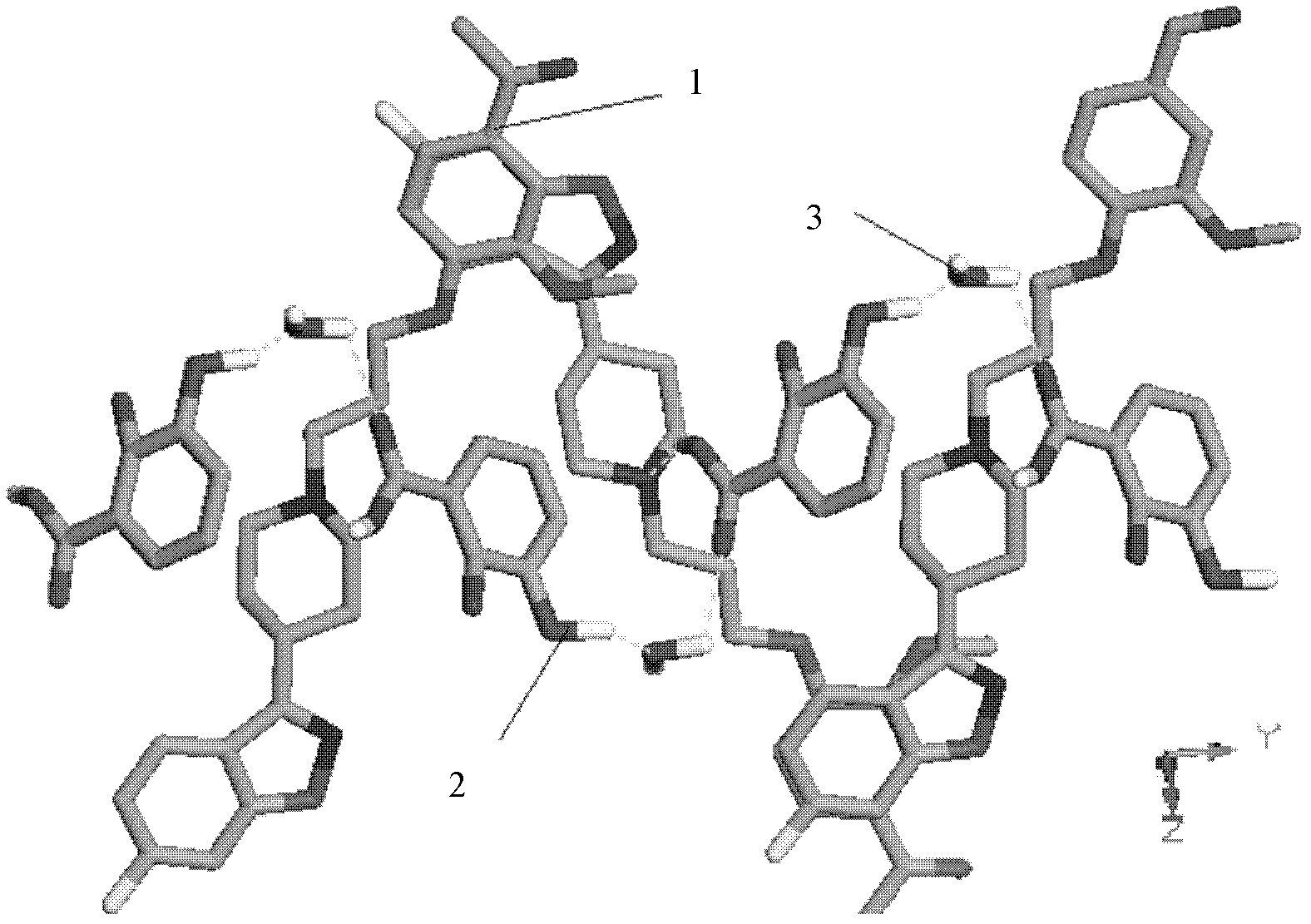
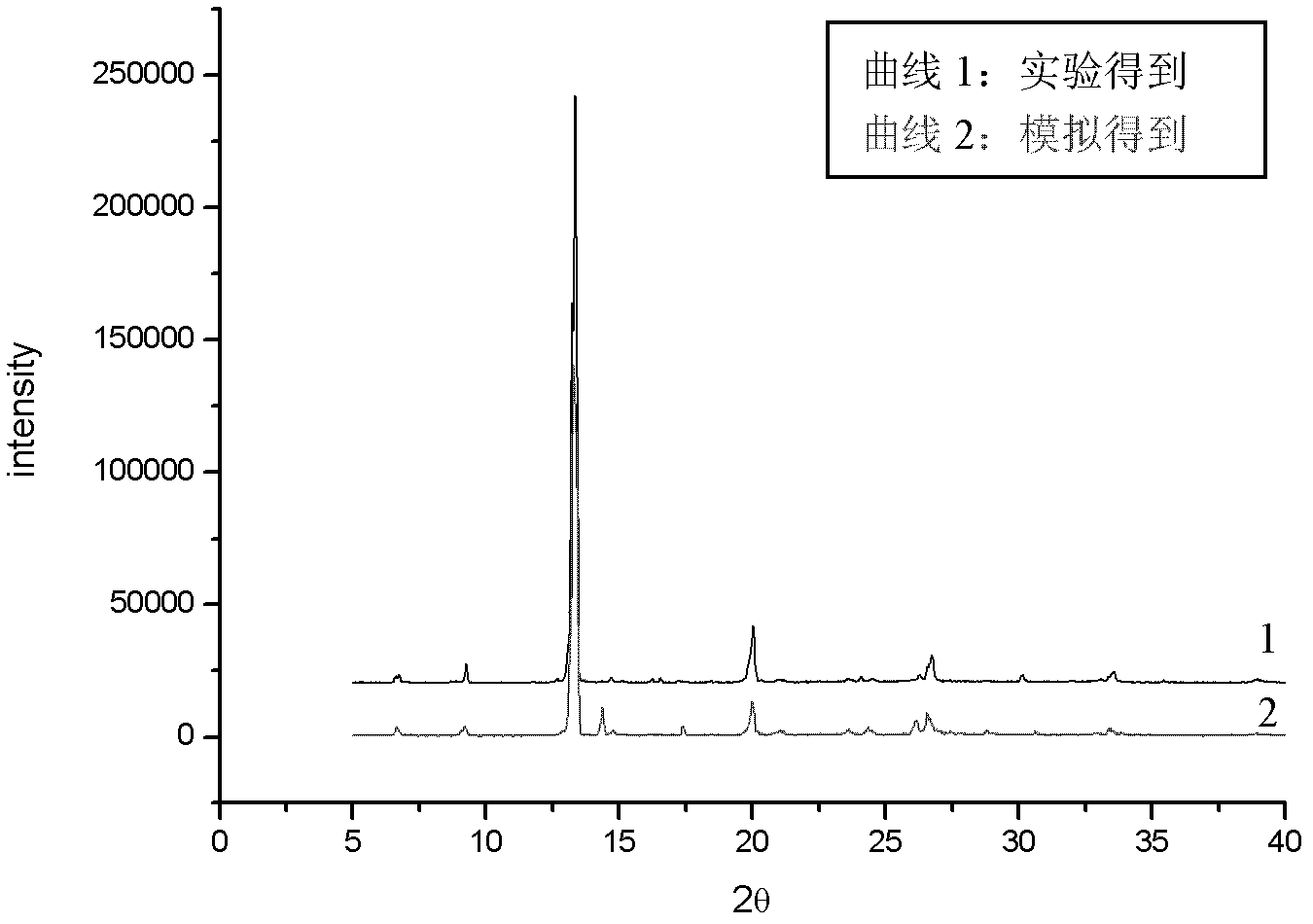
Image
Examples
Embodiment 1
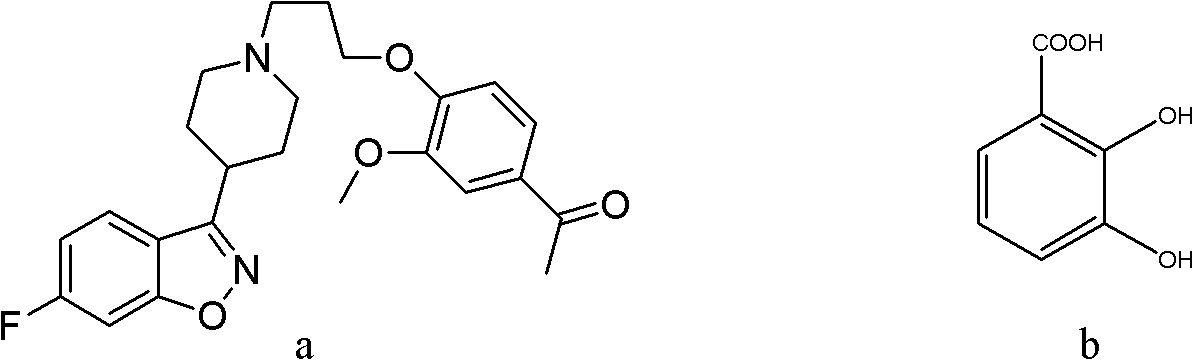
[0029] Synthesis of co-crystals using iloperidone and 2,3-dihydroxybenzoic acid:
[0030] Weighing:
[0031] The reactants are fed according to the mass ratio of iloperidone:2,3-dihydroxybenzoic acid=1:1. Accurately weigh 20.00 mg of iloperidone and 20.00 mg of 2,3-dihydroxybenzoic acid with an analytical balance.
[0032] Dissolution of API:
[0033] Use a 5ml pipette to accurately measure 5ml of ethanol into a 25ml single-necked round bottom flask.
[0034] Reflux-volatilization heat method:
[0035] Put the magnetic stirrer in the round-bottomed flask of the above-mentioned weighed uniformly dissolved medicine, set up the reflux device, the reflux time is 2h, the reflux temperature is 90°C, and the magnetic stirrer and condensed water are turned on.
[0036] After reflux, the reaction solution was filtered, and the filtrate was placed in a 25ml transparent glass vial, and after 6 hours of slow volatilization of the solvent, transparent massive crystals were formed, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com