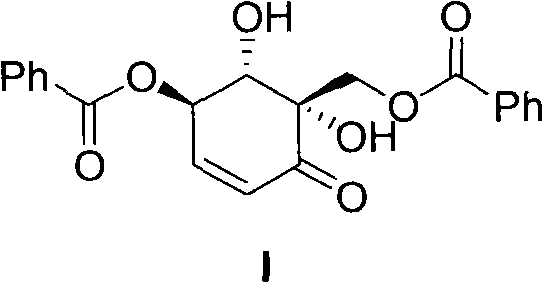
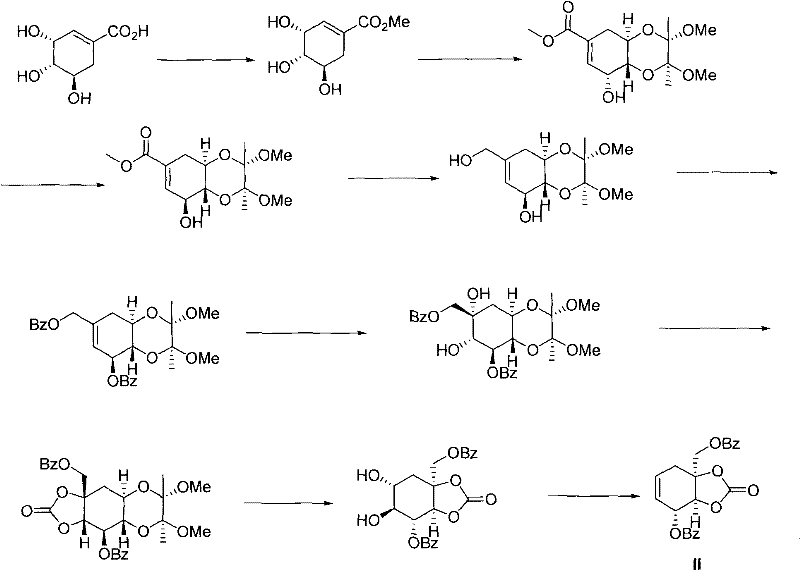
Novel production process for chemical synthesis of zeylenone
A synthetic process and chemical technology, applied in the field of synthetic sansaporenone, can solve the problems of inability, small content, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
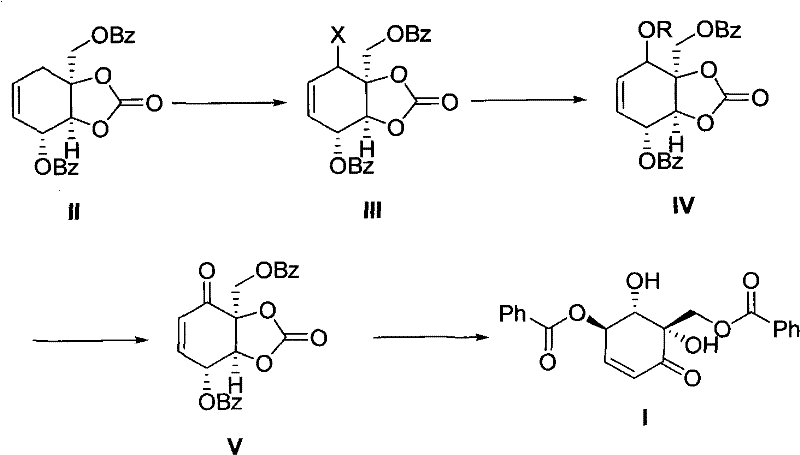
[0015] Embodiment 1 prepares intermediate III:
[0016] Put 50 grams of compound II, 150 grams of n-bromosuccinimide (NBS), and 250 mL of benzene into a 500 mL three-necked round-bottomed flask, and stir at 25°C for 5 hours. The insoluble matter was filtered off, and the solution was extracted three times with water (100 mL×3). The organic phase was dried with anhydrous sodium sulfate, and recrystallized with ethanol to obtain 55 g of light yellow solid compound III. 1 H NMR (400mHz, CDCl 3 ) ppm: 7.98 (m, ArH, 4H), 7.48-7.35 (m, ArH, 6H), 5.61 (m, CH=CH, 2H), 5.01 (m, 1H), 4.82 (s, 1H), 4.60- 4.30 (m, 3H).
Embodiment 2
[0017] Embodiment 2 prepares intermediate IV:
[0018] 50 g of compound III, 150 mL of 1N aqueous sodium hydroxide solution, and 100 mL of methanol were put into a 500 mL three-necked round-bottomed flask and stirred at 55° C. for 10 hours. The reaction mixture was adjusted to pH=4 with 10% citric acid, the precipitated matter was filtered, and the filter cake was washed with water three times (50 mL×3). Air-dried and recrystallized from ethanol to obtain 45 g of compound IV as a white solid. 1 H NMR (400mHz, CDCl 3 ) ppm: 7.97 (m, ArH, 4H), 7.45-7.33 (m, ArH, 6H), 5.59 (m, CH=CH, 2H), 5.00 (m, 1H), 4.81 (s, 1H), 4.58- 4.25 (m, 3H).
Embodiment 3
[0019] Embodiment 3 prepares intermediate V:
[0020] Add 40 g of Compound IV and 150 mL of chloroform into a 500 mL three-neck round bottom flask, and stir at 50°C. Add 9 grams of manganese dioxide every hour for a total of 7 times. After reacting for 10 hours, the insoluble substances were filtered off, the solution was concentrated, separated on a silica gel column, and rinsed with ethyl acetate and petroleum ether to obtain 25 g of white solid compound V. 1 HNMR (400mHz, CDCl 3 ) ppm: 7.97 (m, ArH, 4H), 7.47-7.37 (m, ArH, 6H), 6.75 (m, CH=CH, 1H), 6.35 (m, CH=CH, 1H), 5.22 (m, 1H ), 5.01(s, 1H), 4.68(m, 1H), 4.45(m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com