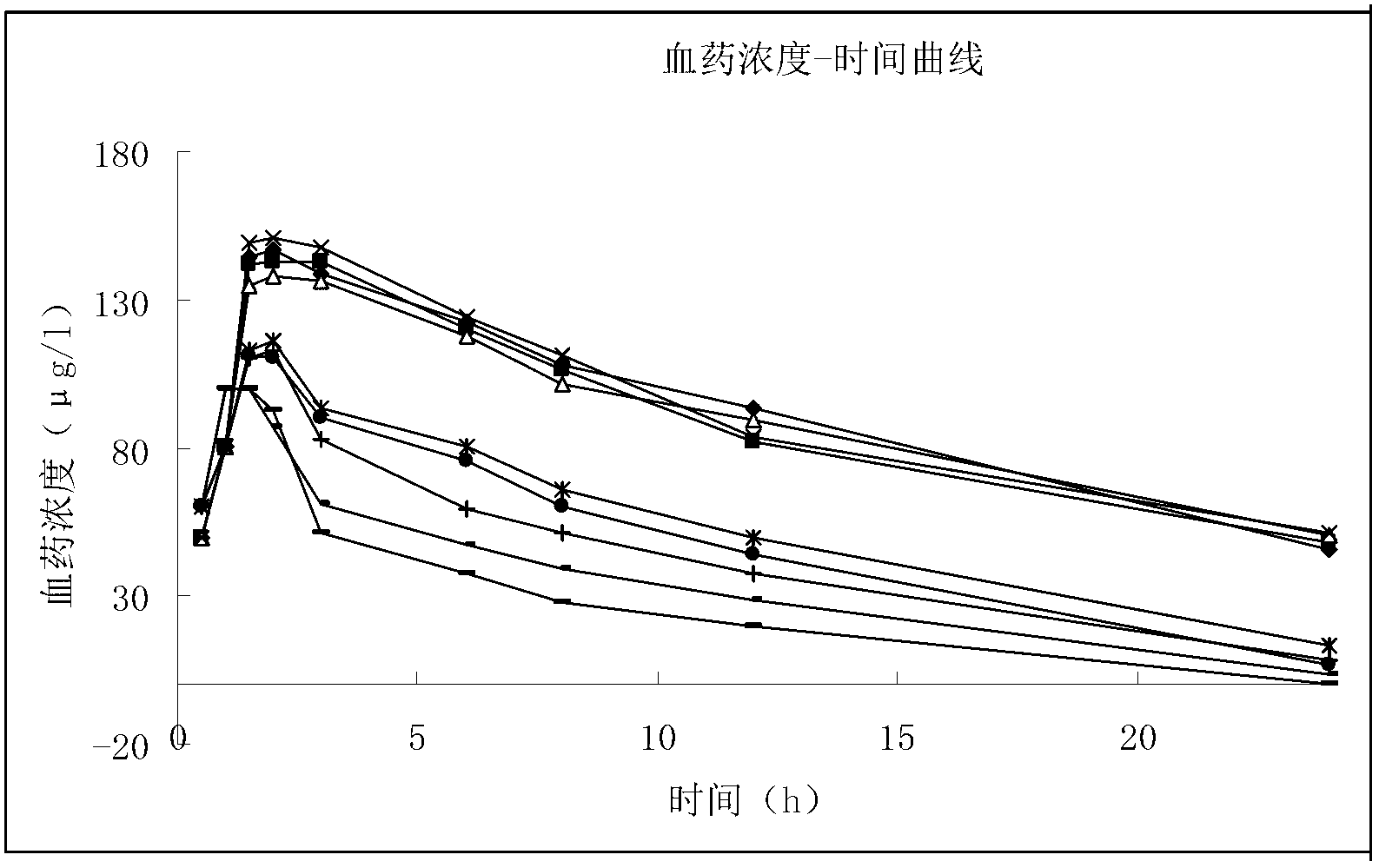
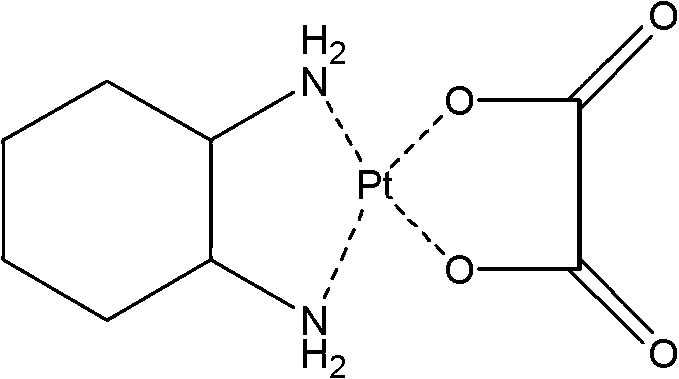
Oxaliplatin vesicular phospholipid gel injection
A gel injection and oxaliplatin technology, applied in the field of pharmaceutical preparations, can solve problems such as long-term stability to be improved, and achieve the effects of improving bioavailability, improving curative effect, and improving long-term stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of Oxaliplatin Vesicular Phospholipid Gel Injection
[0052] Prescription: (100 bottles)
[0053]
[0054] making process:
[0055] (1) Dissolve 10g of oxaliplatin, 170g of soybean lecithin, 50g of cholesterol and 30g of PEG400 in 300ml of methanol solution, stir and sonicate for 30 minutes, and then do gradient homogenization at 200bar to 1000bar for 6 to 7 times to obtain PEG Saliplatin vesicular phospholipid gel solution;
[0056] (2) Add 80g of glucose to the oxaliplatin vesicular phospholipid gel solution prepared above to dissolve, add methanol solution to 500ml and mix well;
[0057] (3) Filtrate with a 0.45 μm microporous membrane, pack in 5 ml / bottle, and quickly freeze and vacuum-dry at -40°C to obtain oxaliplatin vesicular phospholipid gel freeze-dried powder injection.
Embodiment 2
[0058] Example 2 Preparation of Oxaliplatin Vesicular Phospholipid Gel Injection
[0059] Prescription: (100 bottles)
[0060]
[0061] making process:
[0062] (1) Dissolve 5g of oxaliplatin, 100g of soybean lecithin, 30g of cholesterol and 20g of PEG600 in 200ml of methanol solution, stir and sonicate for 30 minutes, and then do gradient homogenization at 200bar to 1000bar for 6 to 7 times to obtain PEG Saliplatin vesicular phospholipid gel solution;
[0063] (2) Add 50g of glucose to the oxaliplatin vesicular phospholipid gel solution prepared above to dissolve, add methanol solution to 400ml and mix well;
[0064] (3) Filtrate with a 0.45 μm microporous membrane, pack in 5 ml / bottle, and quickly freeze and vacuum-dry at -40°C to obtain oxaliplatin vesicular phospholipid gel freeze-dried powder injection.
Embodiment 3
[0065] Example 3 Preparation of Oxaliplatin Vesicular Phospholipid Gel Injection
[0066] Prescription: (100 bottles)
[0067]
[0068] making process:
[0069] (1) Dissolve 10g of oxaliplatin, 140g of soybean lecithin, 40g of cholesterol and 20g of PEG800 in 300ml of methanol solution, stir and sonicate for 30 minutes, and then do gradient homogenization at 200bar to 1000bar for 6 to 7 times to obtain PEG Saliplatin vesicular phospholipid gel solution;
[0070] (2) Add 50g of glucose to the oxaliplatin vesicular phospholipid gel solution prepared above to dissolve, add methanol solution to 500ml and mix evenly;
[0071] (3) Filtrate with a 0.45 μm microporous membrane, and quickly freeze and vacuum dry at -40°C to obtain oxaliplatin vesicular phospholipid gel freeze-dried powder, then add water for injection to 10 L to disperse evenly, and 0.22 μm microporous filter Membrane fine filtration, 100ml / bottle sub-package to obtain oxaliplatin vesicular phospholipid gel inj...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap