Methods and compositions for improved delivery, expression or activity of RNA interference agents
A technology of RNA interference, composition, applied in the direction of DNA/RNA fragments, recombinant DNA technology, botanical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
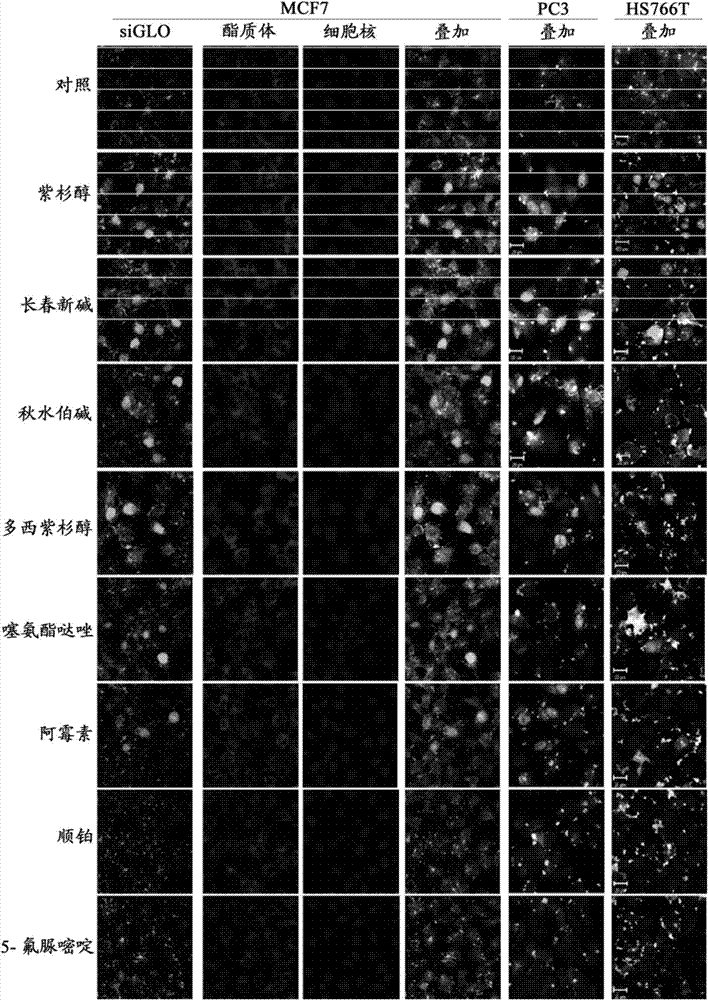
[0204] Preparation of RNAi lipid carrier
[0205]Cationic liposomes were prepared as follows. The desired weight of each lipid was mixed and dissolved in a 90:10 volume ratio mixture of chloroform and methanol. To prepare Pac-PCat-RNAi, add the required amount of paclitaxel to the dissolved lipid. Paclitaxel is added in an amount of 0.04% by weight of the total lipid weight. In the culture medium of a typical in vitro experiment, 0.04% paclitaxel by weight provides a total of 10 nM equivalent concentration of paclitaxel, while 0.2% paclitaxel by weight provides an equivalent concentration of 50 nM. For Doc-PCat-RNAi, Col-PCat-RNAi and Vin-PCat-RNAi, then add appropriate amount of docetaxel, colchicine and vincristine to provide the equivalent concentration of the culture medium as docetaxel 10nM, colchicine 100nM , vincristine 10 nM. For a typical total lipid of 10 mg, 5 mL of chloroform / methanol is required. The dissolved lipids were placed in a round bottom flask and ...
example 2
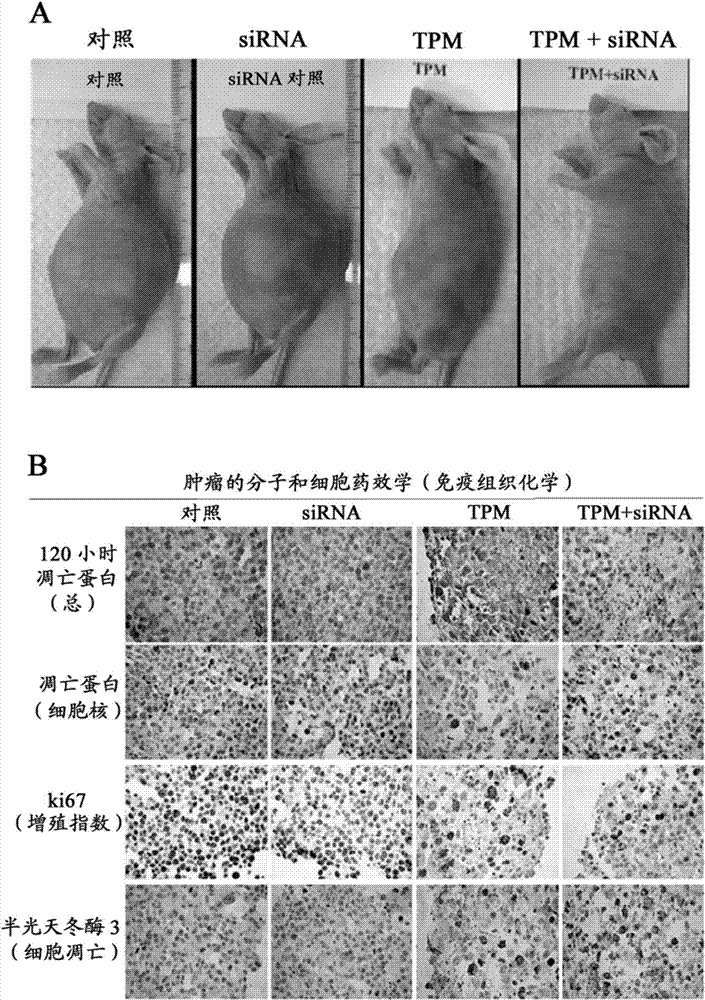
[0208] RIDES plus tumor loosening is effective in vivo: intraperitoneal administration for intraperitoneal tumors
[0209] Experiments were performed in immunosuppressed mice bearing intraperitoneal HS766T pancreatic cancer. Tumor cells were injected into the peritoneal cavity of mice, resulting in tumor formation throughout the peritoneal cavity. In the absence of treatment, the established tumor models eventually resulted in the death of 100% of the animals. Treatment was initiated 10 days after tumor implantation, or half the expected survival time of untreated animals. All treatments were administered intraperitoneally. Day zero is the first treatment and survival time is the time since the first treatment. Paclitaxel was administered in the form of tumor penetrating particles (TPM), prepared as described in US patent application Ser. No. 11 / 242,546. Animals were given a single dose of TPM (immediate-release and sustained-release granules containing a total equivalen...
example 3
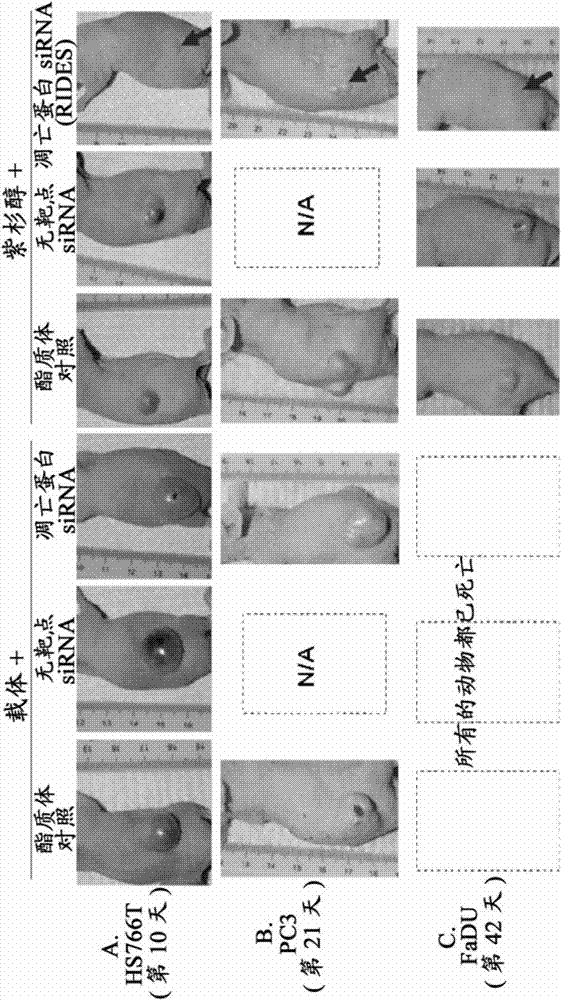
[0219] RIDES works in vivo: intravenous therapy for subcutaneous tumors
[0220] This example demonstrates that intravenously administered RIDES is effective against systemic tumors. The study was performed in immunosuppressed mice bearing subcutaneously implanted human xenograft tumors. Three different types of tumors, namely, pancreatic cancer HS766T, prostate cancer PC3, pharyngeal cancer FaDu. Treatment begins when tumors reach a size greater than 3 mm. Paclitaxel dissolved in polyoxyethylene castor oil 50:50 V / V in ethanol. RNAi is survivin siRNA. The RNAi carrier was PCat liposome, loaded with 1 nmol of siRNA against wild-type survivin mRNA, and 0.12 mg DOTAP per animal. Day zero is the first treatment and survival time is the time from the first treatment.
[0221] Mice bearing HS766T tumors were randomly divided into six groups according to tumor size. Each group consisted of 4 or 5 animals. Treatment begins when tumors reach a size of about 3 mm in diameter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com