Compositions, kits, and methods for identification, assessment, prevention, and therapy of cancer
A technology of cancer, therapy, applied in the field of compositions, kits and methods for identification, evaluation, prevention and treatment of cancer, which can solve the problem of lack of general understanding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0822] [Example 1: Preparation of hydroquinone hydrochloride of 17-AAG]
[0823]
[0824] 17-AAG (0.450 g, 0.768 mmol, 1.0 equiv) was dissolved in dichloromethane (50 mL) and stirred with a 10% aqueous solution of sodium sulfoxylate (50 mL). The solution was stirred for 30 minutes. Collect the organic layer, in Na 2 SO 4 Dry on air, filter and transfer to a round bottom flask. To this solution was added HCl in dioxane (4N, 0.211 mL, 1.1 equiv). The resulting mixture was stirred under nitrogen for 30 minutes. The yellow solid was slowly washed out of solution. The yellow solid was purified by recrystallization from MeOH / EtOAc to yield 0.386 g of hydroquinone HCl salt (2).
[0825] Compound 2 is also referred to herein as IPI-504. IPI-504 (Risspiramycin Hydrochloride) is a water soluble potent inhibitor of Hsp90.
[0826] Additional salts of 17-AAG can be prepared as described herein and / or as known in the art (see, e.g., US 2006 / 0019941, US 7,375,217 and US 7,767,663...
Embodiment 2
[0828] [Example 2: ALK mutation predicts response and clinical benefit of HSP90 inhibitors (including IPI-504) treatment]
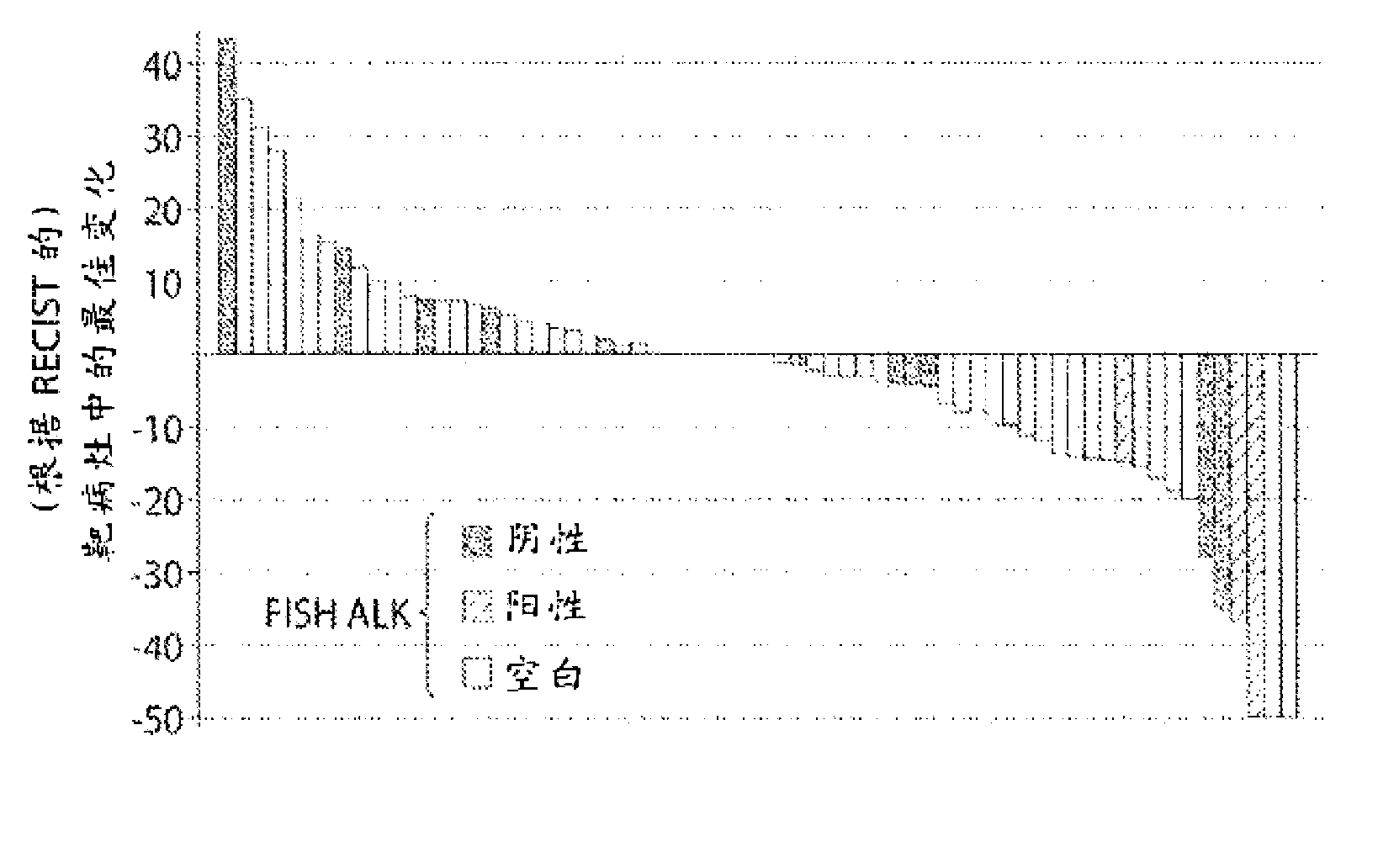
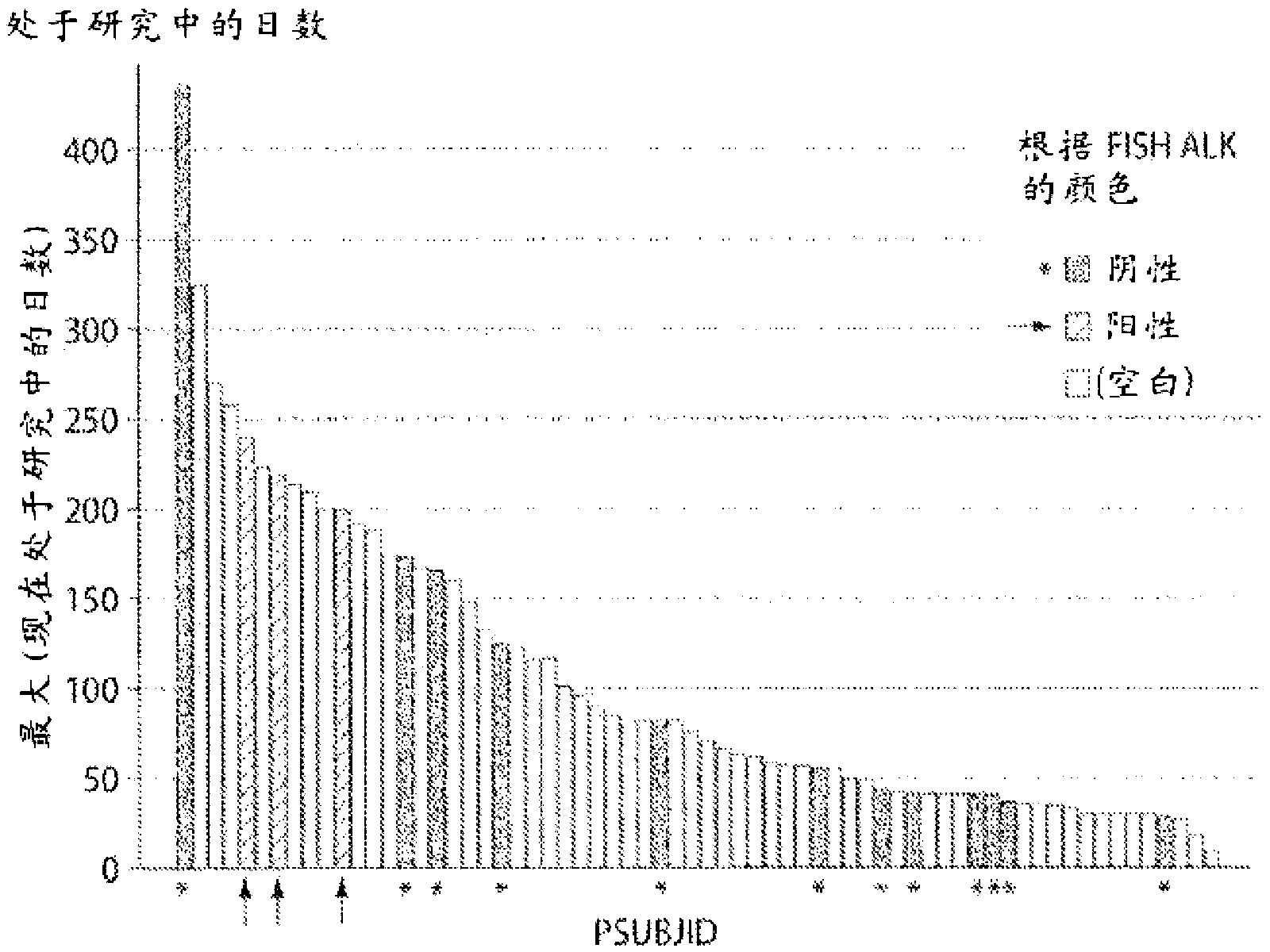
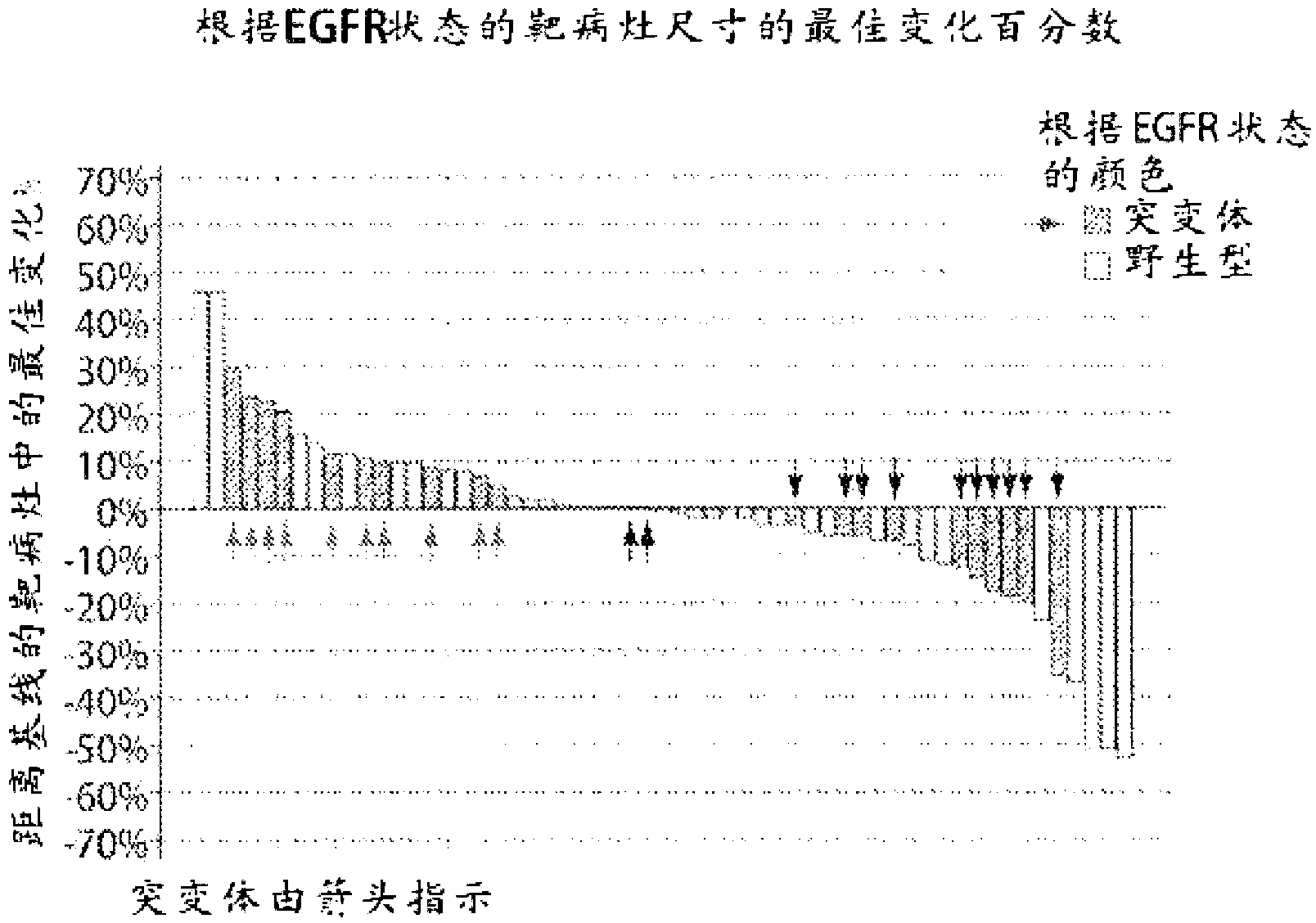
[0829] Retrospective molecular characterization was performed on tumor samples from patients with relapsed and / or refractory stage IIIb or IV non-small cell lung cancer (NSCLC) who were enrolled in a test, IPI-504 (Infinity Pharm. ) Phase 1 / 2 study of safety, tolerability and activity. Use Vysis TM The developed probes were used to determine ALK status by two-color, fracture-separation fluorescence in situ hybridization (FISH) following the manufacturer's protocol. Assays were scored as negative when the probes overlapped (yellow) or were within 2 probe lengths of each other. The assay was scored positive when the probes were separated or the distance between them was greater than 2 probe lengths in >15% of cells or >8 / 50 nuclei. For example, in ALK FISH from a patient with a partial response, wild-type ALK is represented by co-localization of the two ...
Embodiment 3A
[0831] [Example 3A: The relationship between ALK rearrangement (rALK) and the clinical activity of IPI-504 (rhespiramycin hydrochloride) in patients with non-small cell lung cancer (NSCLC)]
[0832] 【Overview】
[0833] This example describes results from a clinical trial evaluating IPI-504, a potent Hsp90 inhibitor described herein, in patients with advanced, molecularly defined non-small cell lung cancer (NSCLC) on EGFR Efficacy after tyrosine kinase inhibitor (TKI) therapy.
[0834] Patients with advanced NSCLC, previously treated with an EGFR TKI and with tumor tissue available for molecular genotyping were enrolled in this prospective, nonrandomized, multicenter, phase II study of IPI-504 monotherapy. The primary outcome was objective response rate. Secondary objectives included safety, progression-free survival (PFS), and activity by molecular subtype analysis.
[0835]Seventy-six patients from ten US cancer centers enrolled between December 2007 and May 2009. An over...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com