One-step production of barium hydroxide and sodium sulfide by using barium sulfide and sodium hydroxide
A barium hydroxide and sodium hydroxide technology, applied in the direction of alkali metal sulfide/polysulfide, calcium/strontium/barium oxide/hydroxide, etc. Product purity impact and other issues, to achieve the effect of saving raw material hydrochloric acid, no pollution to the environment, and shortening the process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
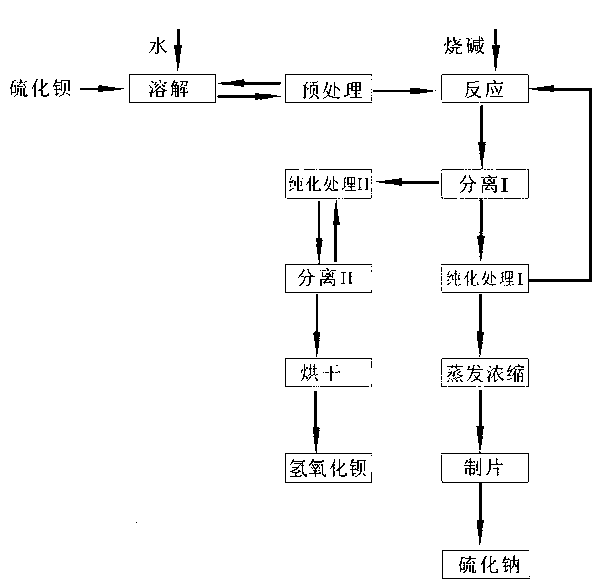
Image
Examples
Embodiment 1
[0026] The industrial barium sulfide black ash that 2kg barium sulfide content is 65% is packed in the container that is used for leaching, adds 3.5L hot water to this container, and the temperature of described hot water is above 95 ℃. During the leaching process, the container can be heated to ensure the water temperature.
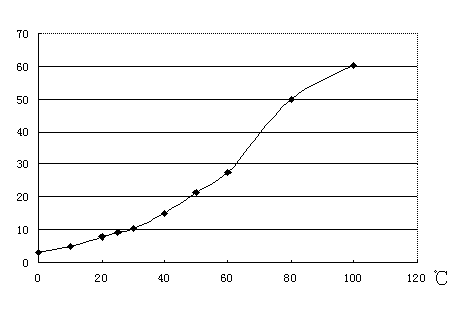
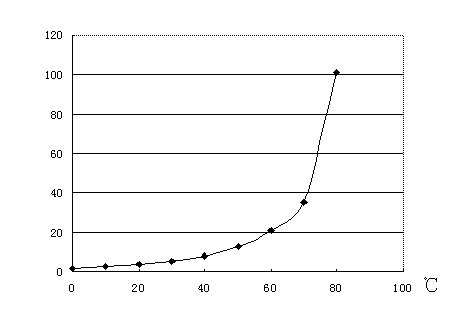
[0027] In the above steps, on the one hand, the leaching of the raw material is repeated until the content of barium sulfide in the raw material residue is lower than 1%. On the other hand, for the leaching solution whose concentration needs to be lower than 20°Be, it is added into the container cyclically as the leaching solution to leach the raw material.
[0028] After the leaching is completed, remove the slag from the leaching solution, add it to the tank, let it stand, keep warm at 95°C, and concentrate to above 30°Be.
[0029] Add the leaching solution after standing for heat preservation into the reaction tank, measure the amount of barium ions ...
Embodiment 2
[0033] The industrial barium sulfide black ash that 2kg barium sulfide content is 55% is packed in the container that is used for leaching, adds 3.5L hot water to this container, and the temperature of described hot water is above 70 ℃. During the leaching process, the container can be heated to ensure the water temperature.
[0034] In the above steps, on the one hand, the leaching of the raw material is repeated until the content of barium sulfide in the raw material residue is lower than 1%. On the other hand, for the leaching solution whose concentration needs to be lower than 20°Be, it is added into the container cyclically as the leaching solution to leach the raw material.
[0035] After the leaching is completed, remove the slag from the leaching solution, add it to the tank, let it stand, keep warm at 80°C, and concentrate to above 30°Be.
[0036] Add the leaching solution after standing for heat preservation into the reaction tank, measure the amount of barium ion i...
Embodiment 3
[0040] The industrial barium sulfide black ash that 2kg barium sulfide content is 60% is packed in the container that is used for leaching, adds 3.5L hot water to this container, and the temperature of described hot water is above 90 ℃. During the leaching process, the container can be heated to ensure the water temperature.
[0041] In the above steps, on the one hand, the leaching of the raw material is repeated until the content of barium sulfide in the raw material residue is lower than 1%. On the other hand, for the leaching solution whose concentration needs to be lower than 20°Be, it is added into the container cyclically as the leaching solution to leach the raw material.
[0042] After the leaching is completed, remove the slag from the leaching solution, add it to the tank, let it stand, keep warm at 90°C, and concentrate to above 30°Be.
[0043] Add the leaching solution after standing for heat preservation into the reaction tank, measure the amount of barium ions ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com