Triple polymerase chain reaction (PCR) kit for duck hepatitis virus type I, duck circoviruses and Muscovy duckling parvovirosis and application of triple PCR kit
A technology for Muscovy duck parvovirus and duck circovirus, applied in the biological field, can solve the problems of long time, low sensitivity, and difficulty in standardization, and achieve the effects of low cost, high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, design and synthesis of primers
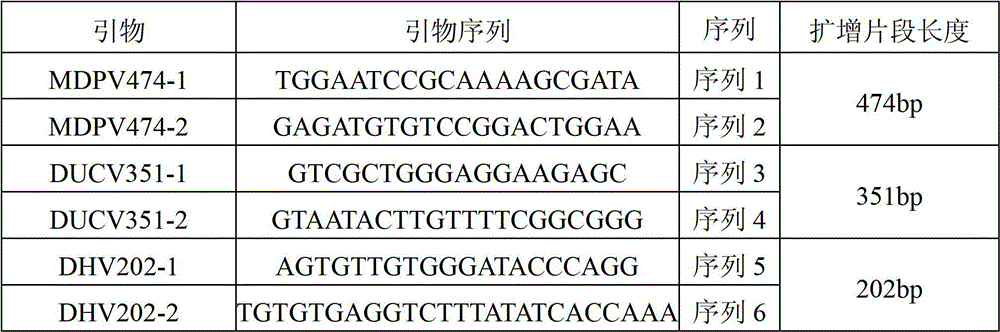
[0039] Three pairs of specific primers (Table 1).
[0040] Table 1 identifies the primer sequences of MDPV, DuCV and DHV I
[0041]
Embodiment 2
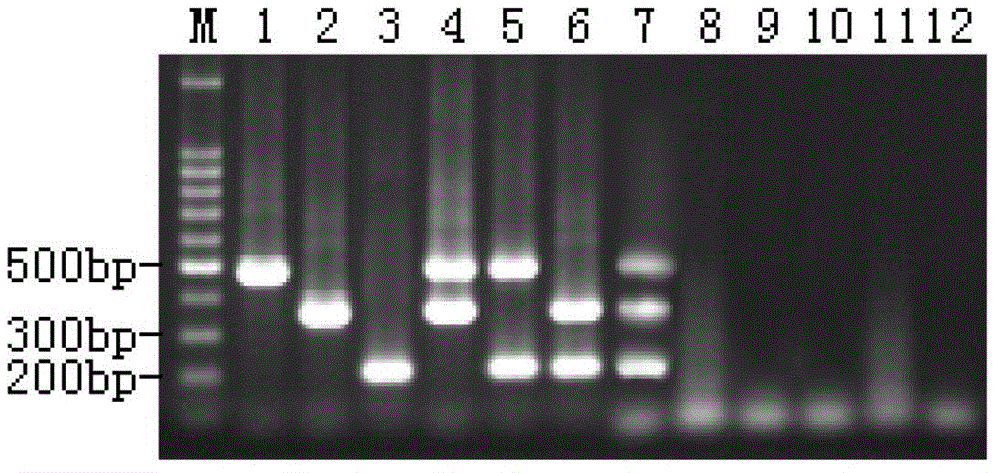
[0042] Example 2, Triple RT-PCR Identification of Duck Type I Hepatitis Virus, Duck Circovirus and Muscovy Duck Parvovirus
[0043] 1. Establishment of triple RT-PCR system
[0044] 1. Preparation of samples to be tested
[0045] According to the instructions of the virus RNA / DNA rapid purification kit, the RNA of the duck type I hepatitis virus AV2111 strain, the DNA of the Muscovy duck parvovirus and the DNA of the duck circovirus were extracted. The concentration and purity of nucleic acid were determined according to the Sambrook method, and stored at -20°C for later use.
[0046] 2. Optimization of triple RT-PCR reaction conditions
[0047] RT-PCR was performed in one step using the One Step RT-PCR Kit. Optimize each cycle parameter and each primer concentration of RT-PCR to determine the best RT-PCR mode.
[0048] By optimizing the concentration of RT-PCR primers, each reaction temperature, time and number of cycles, etc., it was finally determined that the optimal w...
Embodiment 3
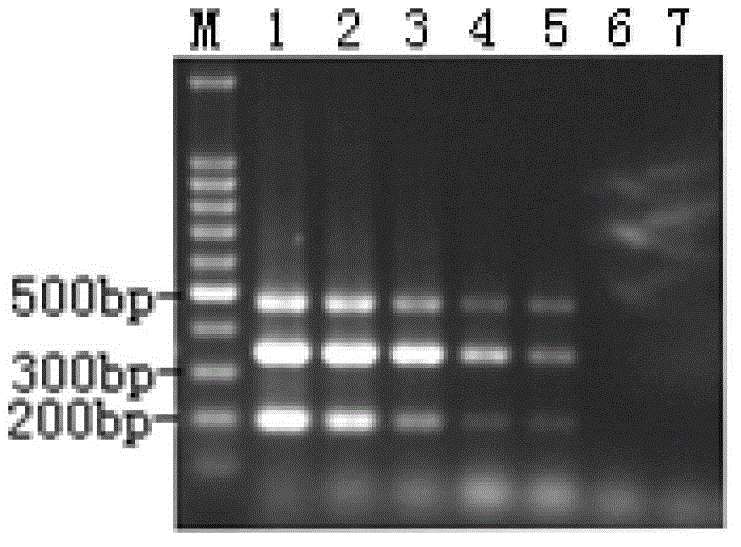
[0064] Embodiment 3, triple RT-PCR detection clinical disease material
[0065] Using the triple RT-PCR rapid detection method for duck type I hepatitis virus, duck circovirus and Muscovy duck parvovirus established in Example 2, 180 disease materials collected in duck groups in Guangxi in 2011 were detected, and the PCR products Clonal sequencing analysis was performed to evaluate its clinical utility.
[0066] 1. Preparation of samples to be tested
[0067] The collected 180 duck disease materials (liver harvesting) were ground into a suspension, and the supernatant was collected by centrifugation after repeated freezing and thawing for 3 times. The DNA and RNA of the duck disease materials were extracted, and 180 samples to be tested were obtained.
[0068] 2. Triple RT-PCR
[0069] The 180 samples to be tested prepared in step 1 were respectively used as templates, and amplified according to the optimized PCR reaction conditions obtained in step 1-2 of embodiment 2.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com