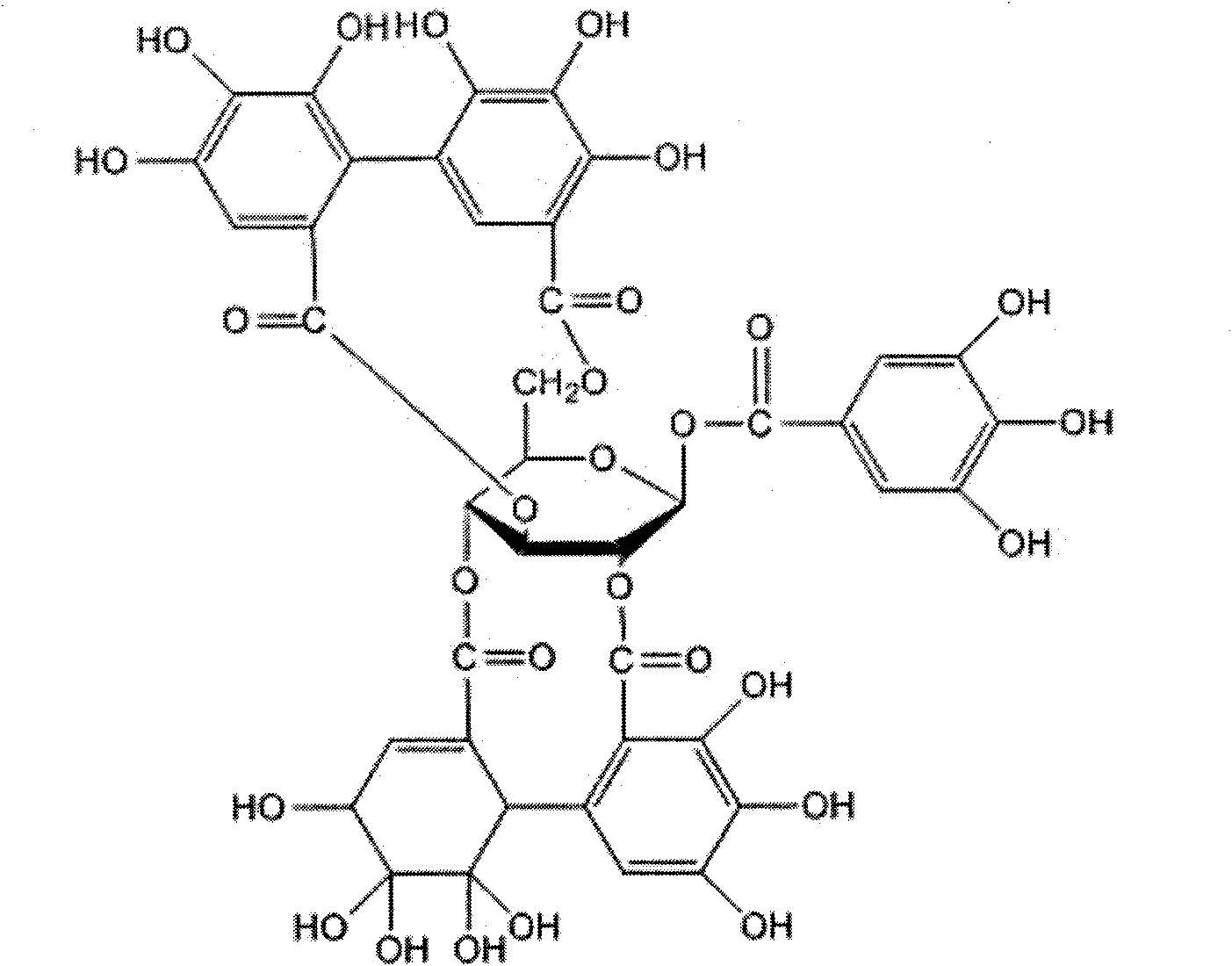
Application of Geraniin extracted from Geranium sibiricum Linne in preparation of anti-herpes virus medicaments
A technology for anti-herpes virus and geranium can be used in anti-viral agents, medical preparations containing active ingredients, pharmaceutical formulas, etc., which can solve the problem of high price of western medicine, little impact on the incubation period and recurrence of herpes virus, and taking cycle. long-term problems, to achieve the effect of good anti-herpes virus activity, low toxicity and resistance to drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
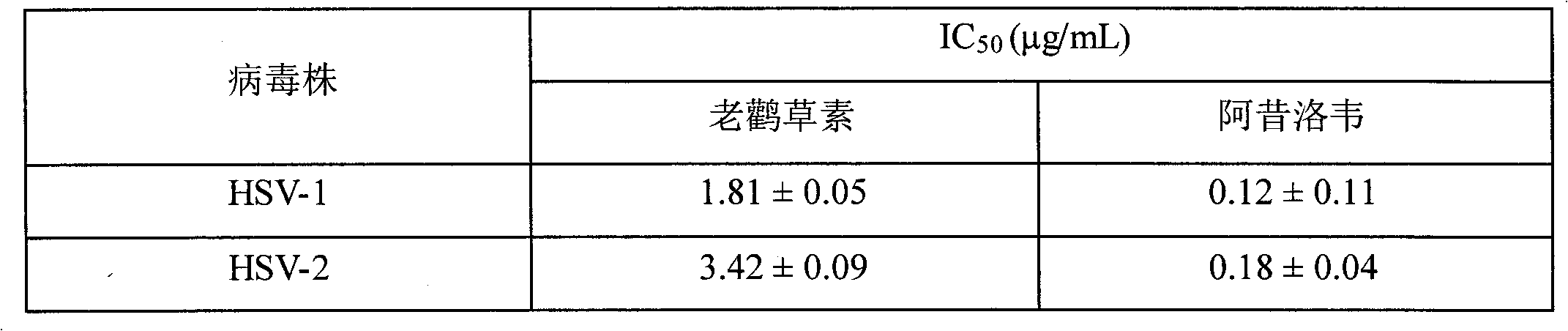
[0022] 1. Cytotoxicity analysis of geraniin extracted from geranium
[0023] (1) Cell culture
[0024] Vero cells were grown in DMEM monolayer medium containing 5% fetal bovine serum and 100 mg / mL penicillin and streptomycin. Cells were passaged at confluence. Cells were placed on 24-well and 6-well plates for cytotoxicity and antiviral analysis, respectively.
[0025] (2) Virus incubation
[0026] HSV-1 and HSV-2 strains were purchased from Wuhan University Virus Research Institute. When the Vero cells grow into a monolayer, the virus is inoculated and placed in CO 2 Cultivate in an incubator, and when the cytopathy reaches more than 90%, put it into a -80°C refrigerator. Combined after repeated freeze-thaw 3 times.
[0027] (3) Cytotoxicity analysis
[0028] A. Place the cells on a 96-well plate and culture at 37°C for 24 hours.
[0029] B. Remove the culture medium, add geraniin containing appropriate dilution, 8 repetitions for each concentration. 200 μL medium co...
Embodiment 2
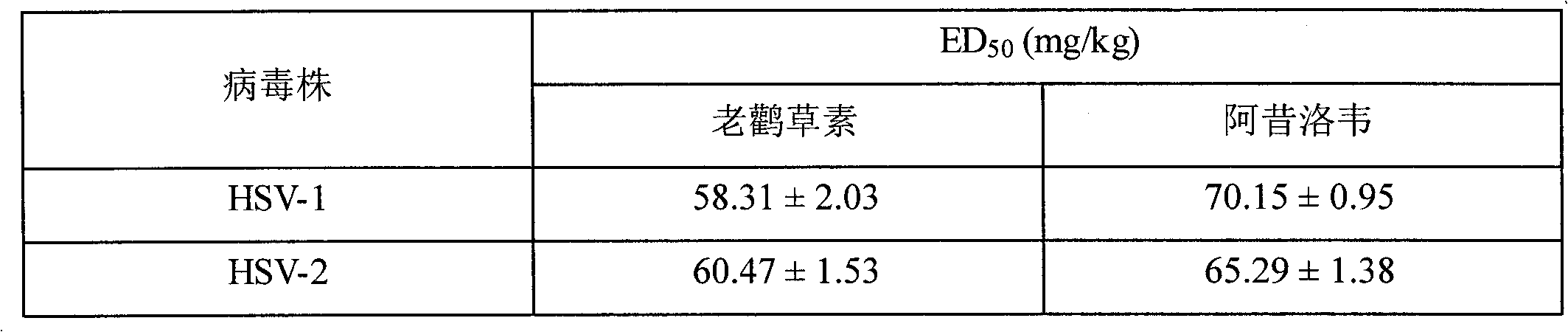
[0052] Example 2: Analysis of anti-herpes virus activity of geraniin extracted from Geranium in animals
[0053] To test the anti-HSV effect of geraniin, 60 mice (provided by the Animal Experiment Center of Harbin Medical University, each weighing 18-22 grams) were needed for the in vivo experiment. Administration high dose group (I), middle dose group (II), low dose group (III), positive control group (IV) and blank control group (V), I, II, III, IV group mice respectively tail vein The injection titer is 0.3mL×10 -8 HSV-1, intragastric administration after fasting overnight (24 hours), group I administration 100mg / kg, group II administration 75mg / kg, group III administration 50mg / kg, group IV administration of normal saline every day, continuous After administration for seven days, the state of the mice was observed every day, the body weight was weighed, and the food was rationed. Fourteen days later, all mice were sacrificed by decapitation and all mice were dissected, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com