Production process for double-layer sterile flexibly-packaged infusion bag
A production process and flexible packaging technology, applied in packaging, wrapping paper, packaging sterilization, etc., can solve the problems of not being able to guarantee uniform penetration of product sterilization temperature, high investment in packaging equipment, and low production efficiency, and achieve low cost and low investment. Less, uniform penetration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
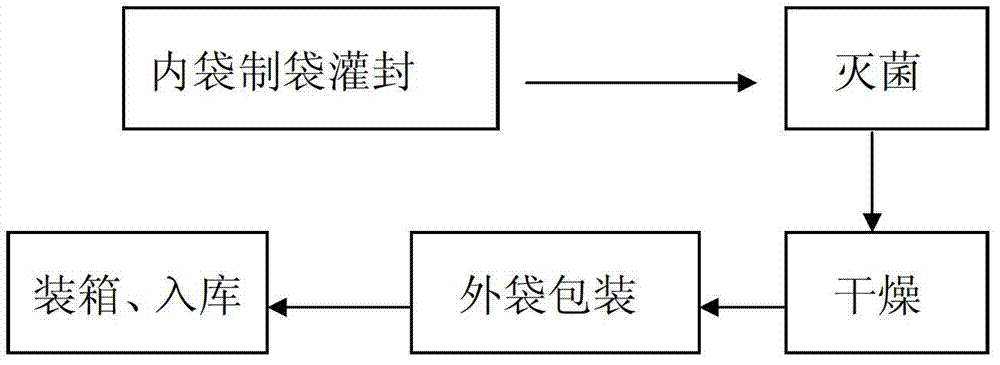
[0019] A production process for double-layer aseptic flexible packaging infusion bags, comprising the following steps:
[0020] (1) Make the infusion film into a bag, fill it with liquid medicine and seal it;
[0021] (2) Put the filled inner packaging bag into the sterilization device for sterilization, the sterilization temperature is 121°C, and the sterilization time is 12 minutes;
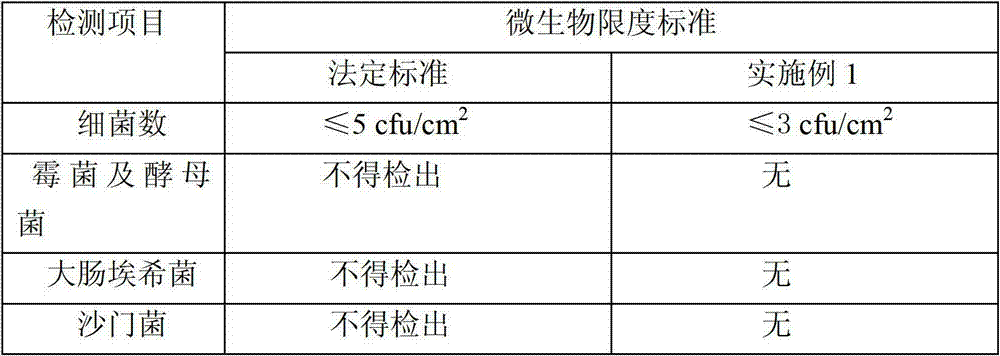
[0022] (3) Blow clean air to the sterilized inner packaging bag to make it dry; the clean air is realized through F6-F7 medium-efficiency air box and H13 high-efficiency filter, and maintain positive pressure. The purpose of sending clean air is to act as a barrier to reduce the risk of pollution. If there is water on the bag, if the clean air is not blown, it is easy to make particles and microorganisms adhere to the surface of the bag and increase the biological load. F6-F7 medium-efficiency fan box and H13 high-efficiency filter are used to ensure that the filtration efficiency reaches 99....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com