Marker for diagnosing liver cancer and application thereof
A technology for liver cancer and its use, applied in the field of oncology and diagnosis, can solve the problems of increased negative proportion of AFP, unsatisfactory sensitivity and specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
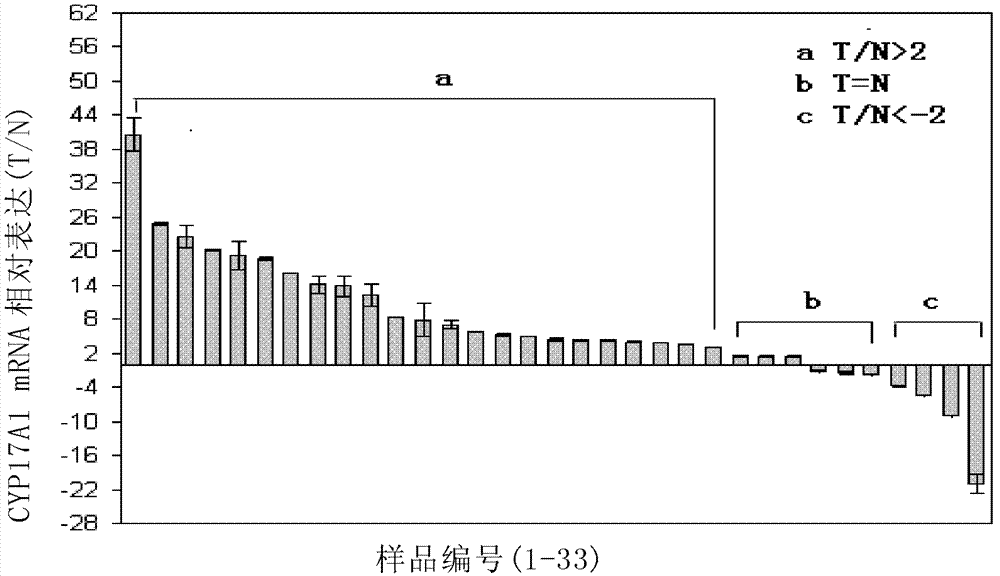
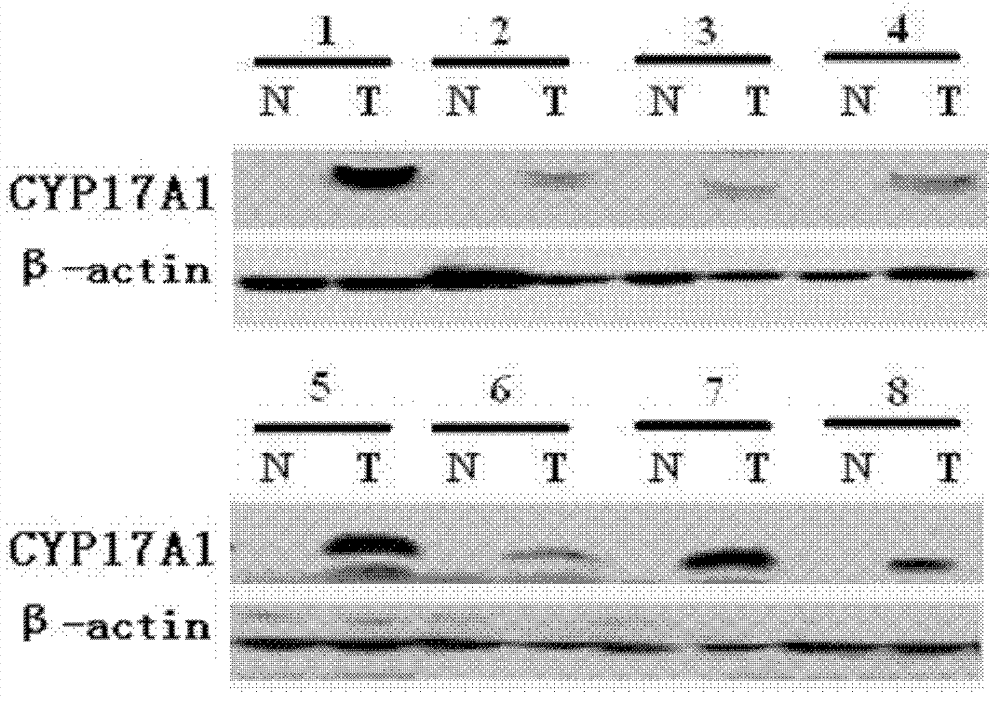
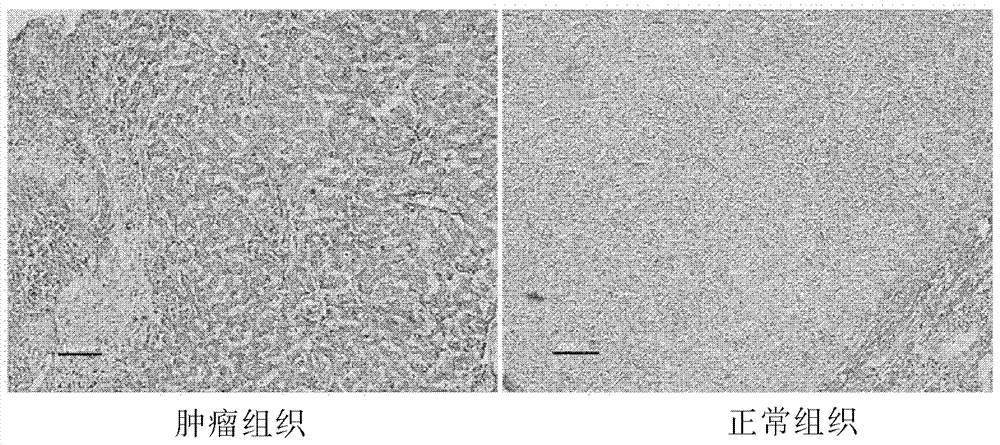
[0082] The above results indicated that, in clinical tissue samples of liver cancer, CYP17A1 mRNA and CYP17A1 protein were highly expressed in liver cancer.
[0083] In addition, the inventors also detected the expression of CYP17A1 in human serum by using enzyme-linked immunosorbent technology. The results showed that the expression of CYP17A1 in the serum of liver cancer patients was significantly higher than that of normal people[ Figure 5 , embodiment 6].
Embodiment 6
[0084] The average content of CYP17A1 protein in serum of normal people (n=30 cases) is 25.5 ng / ml, and the average content of serum of liver cancer patients (n=115 cases) is 115 ng / ml. Statistical analysis showed that there was a significant difference in the high expression of CYP17A1 protein in serum of liver cancer patients (P<0.001).
[0085]According to the 95% confidence interval of CYP17A1 content in normal human serum, when the CYP17A1 concentration is 34.5ng / ml as the cut-off point, the detection sensitivity and specificity can reach 86.1% and 70%, respectively. ROC curve analysis, the results are as follows Figure 5 As shown in B, the larger the area under the ROC curve in the figure, the higher the diagnostic value. The ROC curve area of CYP17A1 is 0.889, which is significantly larger than the reference curve area of 0.5 (P<0.001), indicating that CYP17A1 is a serological molecular marker for liver cancer. It has good diagnostic value.
[0086] sample
[00...
Embodiment 2
[0125] In another preferred embodiment, the present invention also provides a CYP17A1 diagnostic kit, including: a CYP17A1 mRNA diagnostic kit [Example 2] or a CYP17A1 enzyme-linked immunosorbent immunoassay (ELISA) detection kit [Example 7].
[0126] The human liver cancer serological diagnosis kit of the present invention has completed hundreds of experiments, and the positive rate is about 70%.
[0127] The probability of liver cancer of the subjects who are tested positive by the serological diagnostic kit for human liver cancer of the present invention is obviously higher than that of normal population or general liver cancer patients.
[0128] pharmaceutical composition
[0129] The present invention also provides a pharmaceutical composition, which contains the above CYP17A1 antagonist and a pharmaceutically acceptable carrier. The pharmaceutical composition can be used to inhibit the growth of liver cancer cells.
[0130] In the present invention, the antagonist incl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com