Detection kit for c.403>T mutation of CDH23 gene
A kit and gene technology, applied in the field of kits for detection of CDH23 gene mutations, can solve problems such as inability to express, significant impact on protein function, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
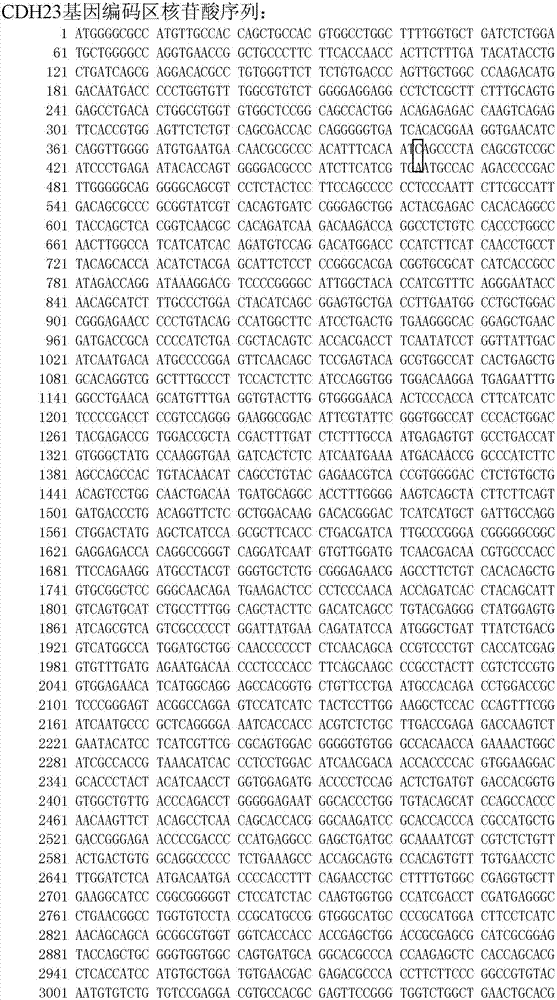
[0067] 【Example 1】 Extraction of blood samples to be tested and PCR amplification of CDH23 gene coding region
[0068] 1. Preparation of blood sample DNA of the subject to be tested
[0069] 1. Research object
[0070] 108 patients with sporadic hereditary deafness and / or Usher 1D syndrome and 100 normal hearing controls without family history were screened for CDH23 gene according to the following method. A CDH23 gene test in a deaf patient was found to be a c.403C>T homozygous mutation. This patient was also accompanied by retinitis pigmentosa and had been diagnosed with Usher 1D syndrome. In his family, the deafness phenotype co-segregated with this genotype. No c.403C>T mutation was found in the screening of 100 normal hearing persons.
[0071] All participants were investigated in detail about their medical history and family history, and a physical examination was performed on them. The otological examination included otoscopy and audiological evaluation. After sig...
Embodiment 2
[0101] [Example 2] Purification and Quantification of PCR Amplified Product of Coding Region of CDH23 Gene
[0102] 1. Purification of PCR products——96-well plate method
[0103] 1. Add 50 μl sterile water to the 96-well plate containing the PCR product and mix well.
[0104] 2. Transfer it to the Millipore purification plate, put it on the vacuum pump for about 3 minutes, and see that there is no water in the purification plate.
[0105] 3. Add 50 μl of deionized water to the purification plate again, and continue to filter until there is no water in the purification plate.
[0106] 4. Remove the purification plate from the vacuum pump, add 20 μl of deionized water to the plate, let it rest for 15 minutes, shake it for another 15 minutes, and then suck it into a new 96-well plate.
[0107] 5. Store in a -20°C refrigerator.
[0108] 2. Quantification by electrophoresis
[0109] 1. Sample preparation
[0110] Take a 96-well spotting plate, add 6 μl of sample buffer to ea...
Embodiment 3
[0121] [Example 3] Direct Sequencing of PCR Amplified Product of Purified CDH23 Gene Coding Region
[0122] 1. Purity and dosage requirements of PCR product DNA template
[0123] DNA purity: OD 260 / OD 280 =1.6~2.0.
[0124] DNA concentration: PCR product 10ng / μl.
[0125] DNA consumption:
[0126] PCR product
[0127]
[0128] 2. Sequencing reaction
[0129] 1. The reagents required for the sequencing reaction should be freshly prepared, and the reagents that need to be sterilized by autoclaving must be sterilized before use. The equipment required for the sequencing reaction (such as 384-well plates, tips, etc.) should also be clean and sterile.
[0130] 2. In order to ensure the freshness of sequencing samples and reaction reagents, it should be operated on ice when adding samples.
[0131] 3. The current reaction system is 5 μl, and the amount of various reagents added is shown in Table 2.
[0132] Table 2 The sequencing reaction system of the PCR amplificati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com