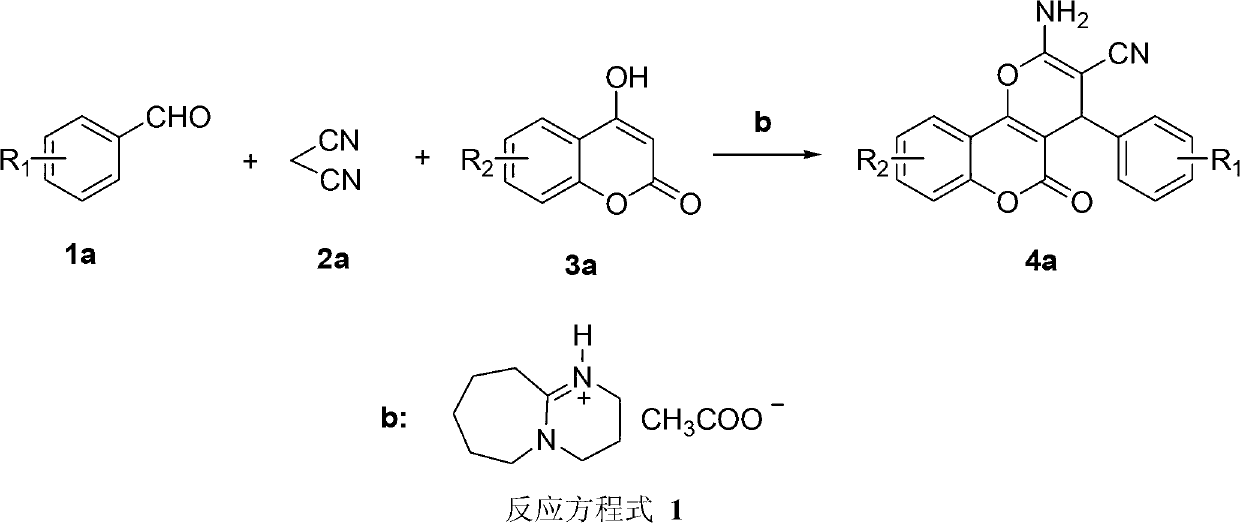
Synthesis method of pyranocoumarin derivatives
A technology of coumarin derivatives and synthetic methods, which is applied in chemical recovery, organic chemistry, etc., can solve the problems of difficult recovery of catalysts, large amount of catalysts, and unfriendly environment, so that the product yield is not affected and the reaction time is not affected. Short, the effect of improving the catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
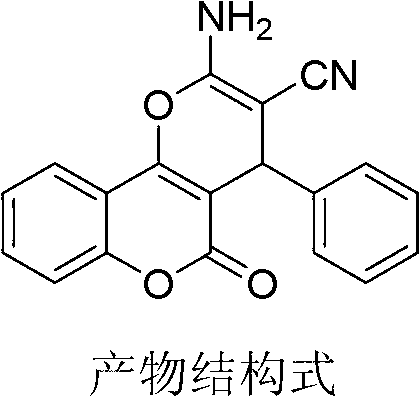
[0022] Add [DBU][Ac] ionic liquid (0.125mmol), benzaldehyde (25mmol), malononitrile (25mmol), 4-hydroxycoumarin (27.5mmol), and deionized water 15mL into the reaction vessel in sequence, Place it in an ultrasonic container, and react ultrasonically at room temperature for 10 minutes (ultrasonic input power 400W), filter the mixture, recrystallize with ethanol, and dry to obtain the pure product with a yield of 94%.
[0023] Characterization data: 1 H NMR (200MHz, DMSO-d 6 ):δ4.45(s,1H),7.23-7.31(m,4H),7.42-7.17(m,4H),7.31(br s,2H),7.40-7.54(m,2H),7.69(t, J=6.4Hz),7.90(d,J=7.8Hz); 13 C NMR (62.5MHz, DMSO-d 6 ) 160.0, 158.7, 154.0, 152.8, 144.0, 133.6, 129.2 (2C), 127.8 (2C), 125.3, 123.1, 119.9, 117.2, 113.6, 104.7, 58.6, 37.6.
Embodiment 2
[0025]
[0026] Add [DBU][Ac] ionic liquid (0.125mmol), 4-chlorobenzaldehyde (25mmol), malononitrile (25mmol), 4-hydroxycoumarin (27.5mmol), and deionized water 15mL to the reaction in sequence Put it in the container, put it in an ultrasonic container, react with ultrasonic wave at room temperature for 10 minutes (ultrasonic input power 400W), filter the mixed solution, recrystallize with ethanol, and dry to obtain the pure product with a yield of 96%.
[0027] Characterization data: 1 H NMR (200MHz, DMSO-d 6 ):δ4.49(s,1H),7.29-7.40(m,4H),7.49(br s,2H),7.54-7.74(m,2H),7.88-8.55(m,2H); 13 C NMR (62.5MHz, DMSO-d 6 ): 160.1, 158.6, 154.2, 152.9, 142.9, 133.6, 129.1 (2C), 127.8 (2C), 125.3, 123.2, 119.9, 117.2, 113.6, 104.1, 58.2, 37.1.
Embodiment 3
[0029]
[0030] Add [DBU][Ac] ionic liquid (0.125mmol), 3-chlorobenzaldehyde (25mmol), malononitrile (25mmol), 4-hydroxycoumarin (27.5mmol), and deionized water 15mL to the reaction in sequence Put it in the container, put it in an ultrasonic container, react with ultrasonic wave at room temperature for 10 minutes (ultrasonic input power 400W), filter the mixed solution, recrystallize with ethanol, and dry to obtain the pure product with a yield of 90%.
[0031] characterizing data 1 H NMR (200MHz, DMSO-d 6 ):δ4.51(s,1H),7.26-7.35(m,4H),7.45(br s,2H),7.47-7.54(m,2H),7.68-7.76(m,2H),7.78-7.82( m,2H); 13 CNMR (62.5MHz, DMSO-d 6 ): 160.2, 158.7, 154.4, 152.9, 146.5, 134.3, 132.0, 129.3, 128.3, 127.9, 127.3, 125.3, 123.1, 119.7, 117.2, 113.7, 103.9, 58.1, 37.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com