Preparation method of optically pure cis-2-(diphenylphosphine)-1-cyclohexanecarboxylic acid
A technology of diphenylphosphine and cyclohexanecarboxylic acid, applied in the field of preparation of optically pure cis-2--1-cyclohexylcarboxylic acid, to achieve the effect of improving yield and purity and promoting catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of the first intermediate
[0028] at room temperature and N 2 Under protection, 1.76g(R)-H 8 -BINOL, 72 μL H 2 O was added to 40mL of anhydrous tetrahydrofuran, stirred vigorously at room temperature for 5min, 4mmol of dibutylmagnesium dissolved in heptane was added dropwise to the reaction system, and then 8.1g of diphenylphosphine oxide was added to the reaction system, Stir vigorously at room temperature for 5 minutes. After the stirring is completed, the reaction system is cooled to -20° C., and kept at this temperature for 5 minutes. Methyl cyclohexenecarboxylate (5.88 g, 42 mmol) is added dropwise to the reaction system. After the addition is completed, - React at 20°C for 48 hours; after the reaction, quench the reaction with saturated ammonium chloride solution, remove the solvent under reduced pressure, extract the aqueous phase with chloroform three times, dry the extracted organic phase with anhydrous sodium sulfate, remove th...
Embodiment 2
[0038] Embodiment 2: the preparation of the second intermediate
[0039] at room temperature and N 2 Under protection, 10.38g, 30mmol of the first intermediate was dissolved in 45mL of anhydrous toluene, stirred at room temperature, the reaction system was cooled to 0°C, 12mL, 90mmol of triethylamine was added dropwise to the reaction system, and 8.5mL, 60mmol trichlorosilane, a white precipitate will form when trichlorosilane is added, the mixture is stirred at 100°C for 18 hours, then the resulting suspension is transferred to an ice bath to cool, and the 2 Quenched with degassed 100mL 10% NaOH aqueous solution, then filtered with a funnel to remove the white solid, washed with toluene, extracted the aqueous phase with 100mL×3 dichloromethane, combined the organic phases and dried them over anhydrous sodium sulfate. The solvent was removed under reduced pressure to obtain a crude product, and column chromatography was carried out, eluent: n-hexane:ethyl acetate=50:1~20:1, t...
Embodiment 3
[0049] Example 3: Pure cis 2-(diphenylphosphine)-1-cyclohexanecarboxylic acid
[0050] at 0°C and N 2 Under protection, 27 mL of dimethyl sulfide was added to 1.63 g, 5 mmol of the second intermediate and 6.0 g, 25 mmol of aluminum trichloride, and then the mixture was stirred at room temperature overnight. Then the mixture was cooled to 0 °C, the reaction system was added dropwise into 30 mL of ice water, and then quickly extracted with 30 mL × 3 dichloromethane, the combined organic phase was dried with anhydrous sodium sulfate, N 2 Atmosphere, filter at 0°C and remove the solvent under reduced pressure to obtain 1.3 g of pure cis-2-(diphenylphosphine)-1-cyclohexanecarboxylic acid, yield 83%. All the above solvents were cooled in advance at -20°C and washed with N 2 Degassing treatment.
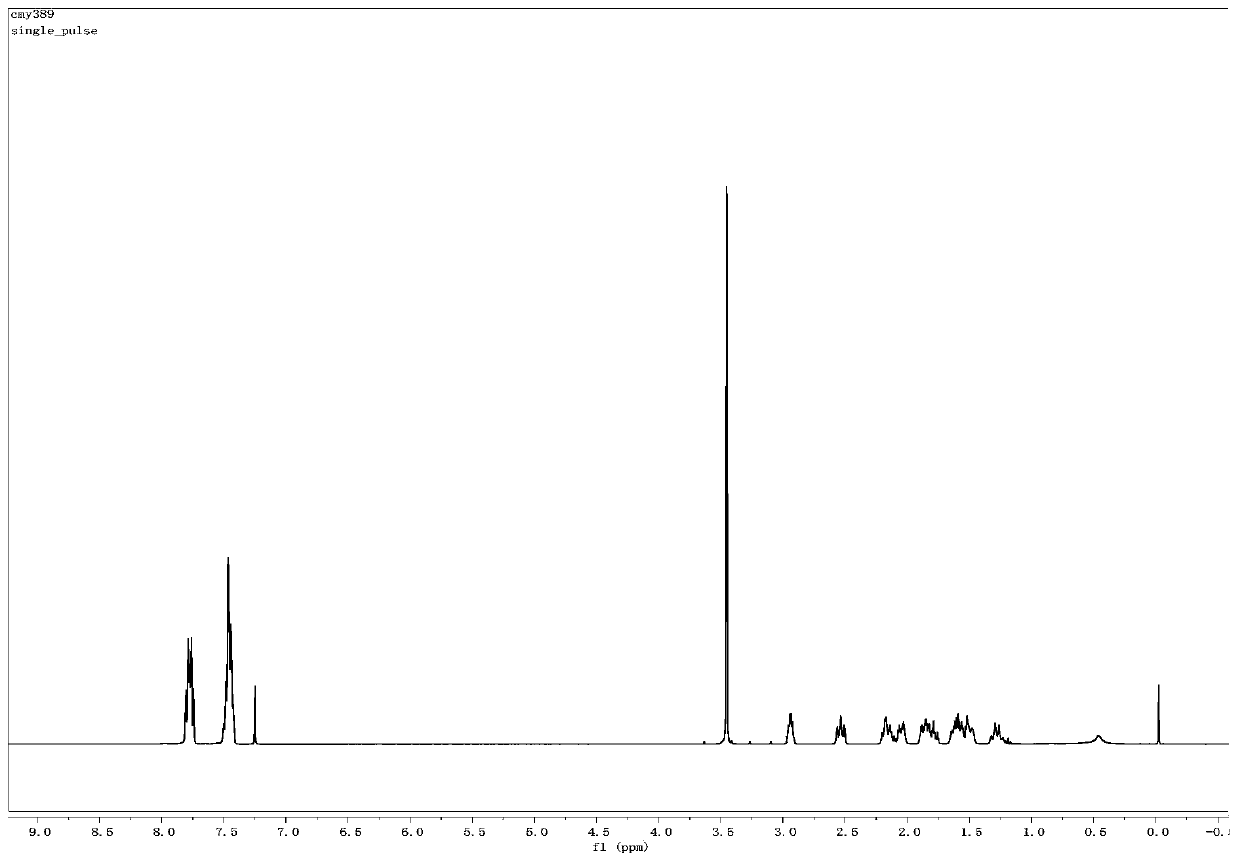
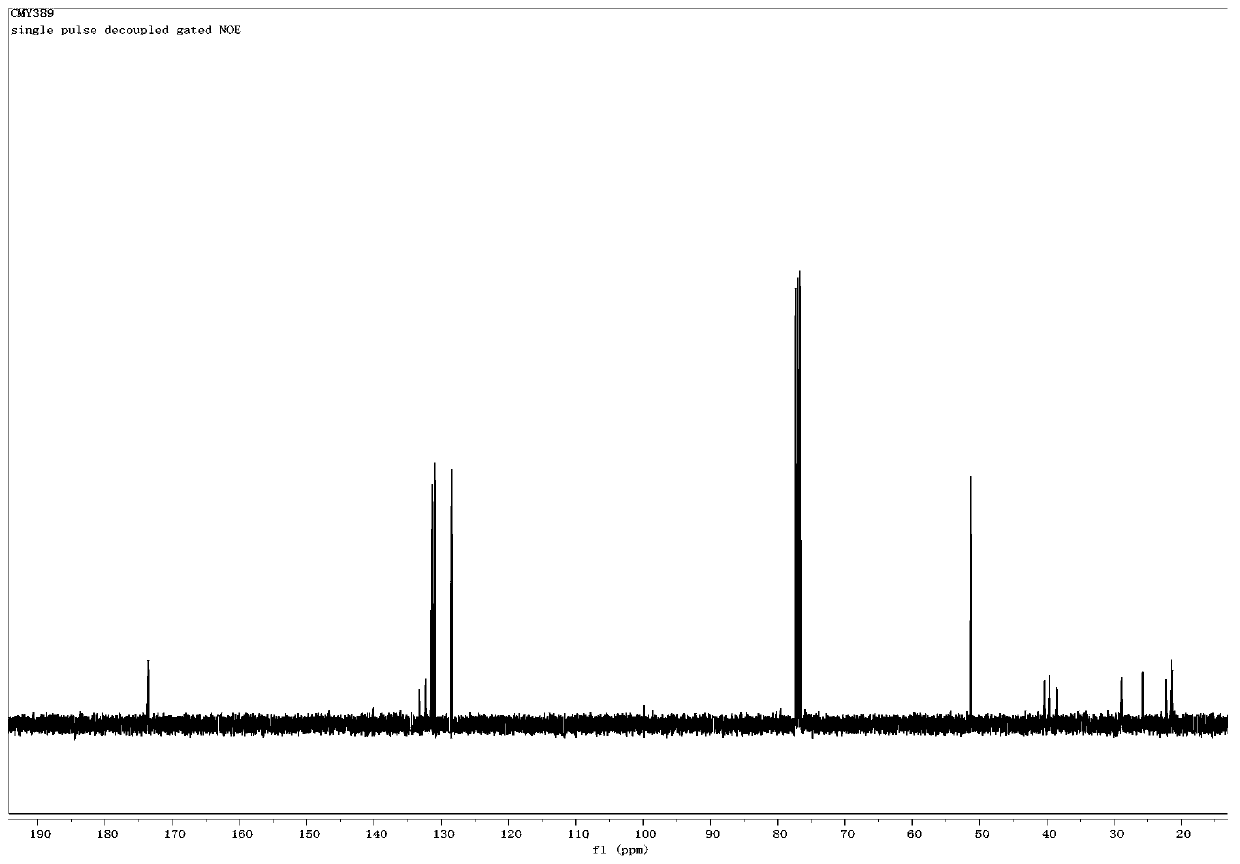
[0051] The structural characterization data of the product obtained in embodiment 3 are as follows:
[0052] 1 H NMR (400MHz, CDCl 3 ):δ7.68-7.52(m,4H),7.39-7.30(m,6H),2.67-2.51(m,2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com