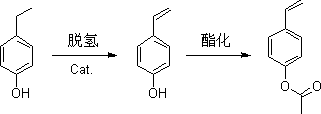
Preparation method of p-acetoxystyrene and preparation method of its intermediate
A technology of acetoxystyrene and acetoxyacetophenone, which is applied in the field of preparation of p-acetoxystyrene, can solve the problems of high equipment requirements, industrialization is not feasible, and raw materials are not easy to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] The invention discloses a preparation method of p-acetoxystyrene and a preparation method of an intermediate thereof. Those skilled in the art can refer to the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0092] The reagents used in the preparation method of p-acetoxystyrene and the preparation method of its intermediate provided by the present invention ...
Embodiment 1
[0094] Embodiment 1 Preparation of p-acetoxystyrene
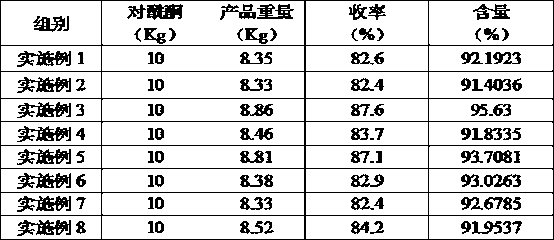
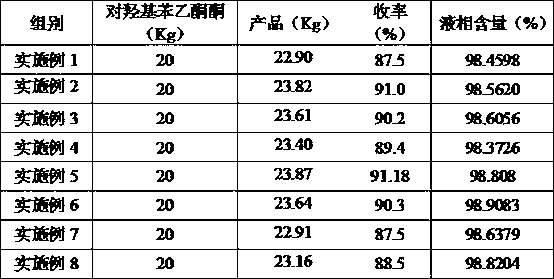
[0095] Accurately weigh 20 kg (0.15 mol) of p-hydroxyacetophenone and 15.3 kg (0.15 mol) of acetic anhydride for acetylation reaction, the reaction temperature is 180°C, and the reaction time is 2 hours. Distill under reduced pressure at a pressure of 0.1mmHg, collect fractions at 60-63°C, and obtain p-acetoxyacetophenone;
[0096] Accurately weigh 10kg of p-acetoxyacetophenone, melt it at 70°C, add 5 kg of hydrogenation solvent methanol (mass ratio: 1:0.5) A hydrogenation reaction occurs under catalysis, and distilled under a pressure of 0.1mmHg, and the fraction at 80-85°C is collected to obtain p-acetoxybenzylmethanol;
[0097] Accurately weigh 20 kg of p-acetoxybenzyl methanol in the presence of 0.02 kg of dehydrating agent hydrochloric acid (mass ratio 100:0.1), and 0.2 kg of polymerization inhibitor alkylhydroxybenzenesulfonic acid compounds (mass ratio 100: 1) , dehydrated at 145°C for 30h, collected 135°C fractio...
Embodiment 2
[0098] Embodiment 2 Preparation of p-acetoxystyrene
[0099] Accurately weigh 20 kg (0.15 mol) of p-hydroxyacetophenone and 153.14 kg (1.5 mol) of acetic anhydride for acetylation reaction, the reaction temperature is 80°C, and the reaction time is 30 h. Distill under reduced pressure at a pressure of 760mmHg, collect fractions at 326-330°C, and obtain p-acetoxyacetophenone;
[0100] Accurately weigh 10kg of p-acetoxyacetophenone, melt it at 50°C, add 300kg of hydrogenation solvent n-propanol (mass ratio: 1:30) A hydrogenation reaction occurs under catalysis, and after rectification under a pressure of 760mmHg, the fraction at 323-330°C is collected to obtain p-acetoxybenzylmethanol;
[0101] Accurately weigh 20 kg of p-acetoxybenzylmethanol in 0.02 kg of dehydrating agent hydrogen bromide (mass ratio 100: 0.1), 0.02 kg of polymerization inhibitor alkylhydroxybenzene sulfonic acid compounds, hydroxylamine compounds (mass ratio 100: 0.1) 0.1) Dehydration at 160°C for 2 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com