Drug combination product for analgesia based on sinomenine
A combination product, the technology of sinomenine, which is applied in the field of new analgesic drug combination products, can solve the problems of analgesic synergy that have not been reported yet, and achieve the effects of preventing drug tolerance, reducing side effects, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synergistic analgesic effect of sinomenine and gabapentin in a mouse model of peripheral neurogenic pain
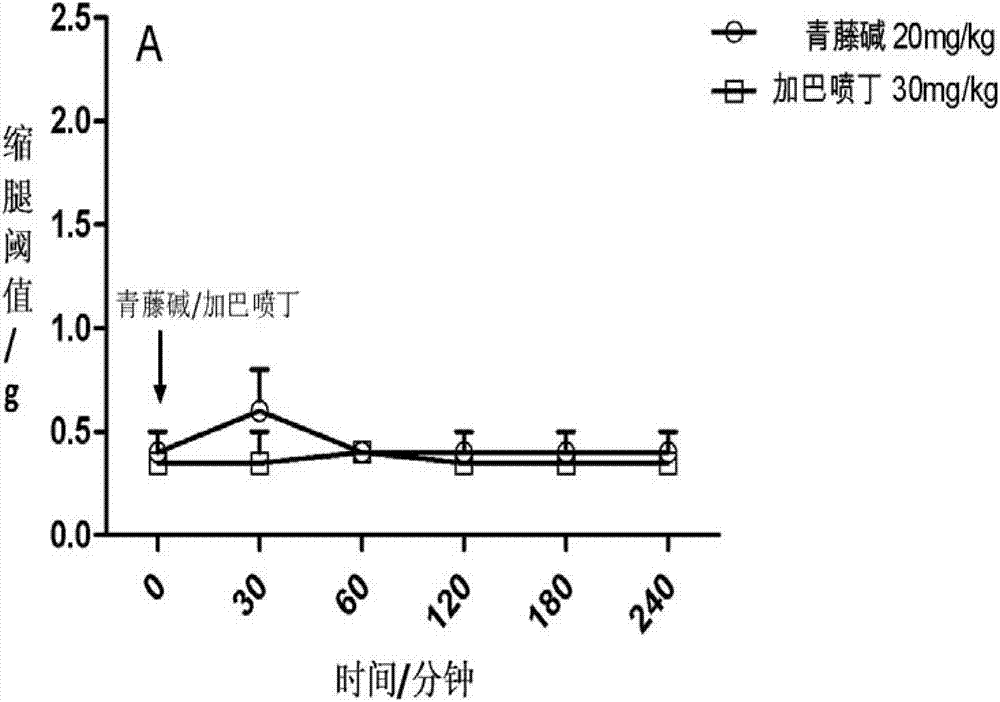
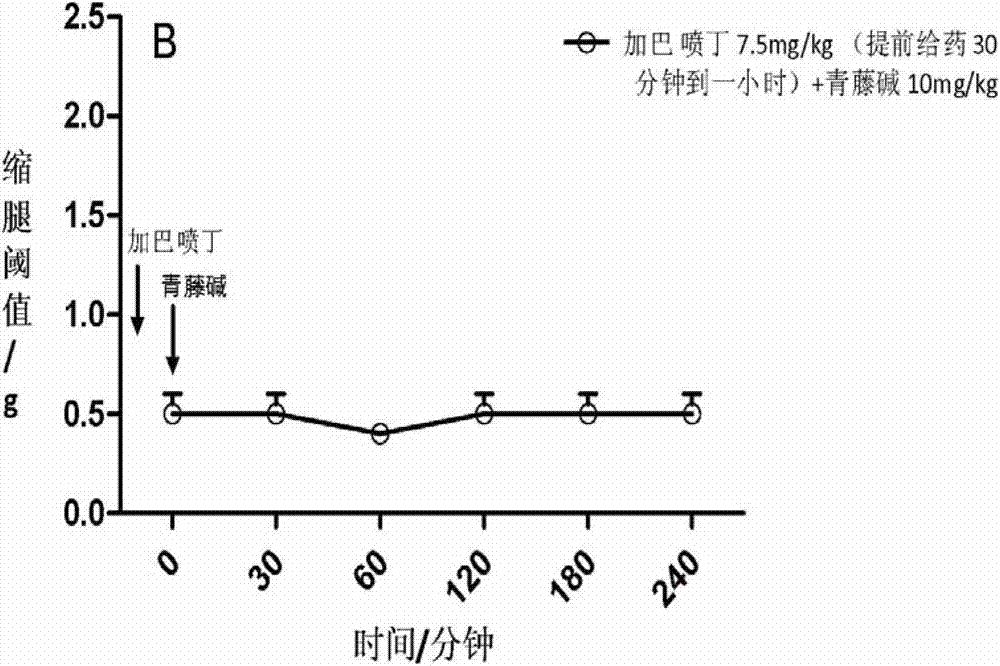
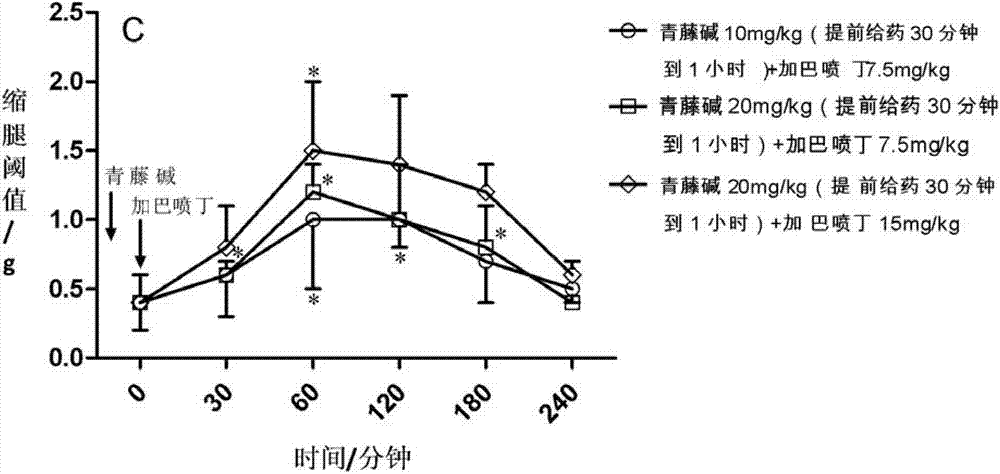
[0049] In a mouse model of partial sciatic nerve injury, mechanical hyperalgesia can be observed, manifested as a decrease in the response threshold to mechanical stimuli. The sinomenine of 20mg / kg mouse body weight was administered orally alone and within 0.5 to 4 hours after administration (observed once every 30 minutes to 60 minutes), the leg withdrawal threshold (g) of the mice was observed, and the results showed no analgesic effect ( See Figure 1A ); gabapentin administered 30 mg / kg body weight of mice orally alone and within 0.5 to 4 hours after administration (observed once every 30 minutes to 60 minutes) observed the mouse's leg withdrawal threshold, the results showed no analgesic effect (see Figure 1A ). When sinomenine and gabapentin are administered together in small doses, first administer 7.5 mg / kg of gabapentin, and then administer 10 mg / kg of ...
Embodiment 2
[0051] Synergistic analgesic effect of sinomenine and gabapentin in a rat model of spinal cord injury
[0052] In the pain model of spinal cord injury in rats, strong pain sensitivity to mechanical and cold stimuli can also be observed. Single administration (intraperitoneal injection) of 20 mg / kg rat body weight of sinomenine or single administration of 30 mg / kg rat body weight of gabapentin, within 0.5 to 24 hours after administration, the screaming threshold (g) and cold Stimulation under the response to give points (rat p-chloroethane spray ( Europa AB, Sweden) responds to the cold stimulation that pain-sensitive parts cause on its body and the standard is: 0=no observable response; 1=partial reaction (skin twitching and muscle contraction), without screaming; 2=temporary short screaming and evasion behavior; 3=sustained screaming and evasion behavior), the results show that there is no analgesic effect of these two agents administered alone in this model (see Figure 2...
Embodiment 3
[0056] Chronic synergistic analgesic effect of sinomenine and gabapentin
[0057] In the pain model of spinal cord injury in rats, the combination of sinomenine at 10 mg / kg rat body weight and gabapentin at 4 mg / kg rat body weight was selected to study the effect of chronic administration. In two rounds of experiments, combined administration (intraperitoneal injection) twice a day, each administration was administered 10 mg / kg sinomenine 30 minutes to 1 hour followed by 4 mg / kg gabapentin, and in which the first administration The baseline screaming threshold (ie pain threshold) was recorded before, and the screaming threshold was recorded 2 hours after the second administration, with an interval of 6 hours between the two administrations. The result is as Figure 4A with 4B Shown, combined administration in 7 days ( Figure 4A ) or 14 days ( Figure 4B ) produced obvious analgesic effect, and no tolerance phenomenon occurred. More importantly, in the process of chronic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com