Preparation method of methoxylphenyl propanol substituted 6-amino-beta-cyclodextrin derivative
A technology of methoxyphenylpropanol and methoxyphenyl, which is applied in the field of preparation of 6-amino-β-cyclodextrin derivatives substituted by methoxyphenylpropanol, which can solve the limitations, limited water solubility, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
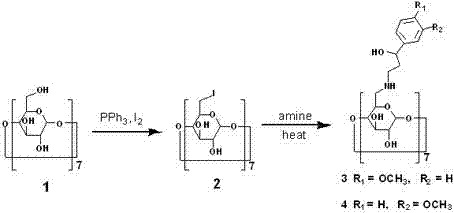
[0010] 6-iodo-6-deoxy-β-cyclodextrin ( 2 ) preparation
[0011] Starting from β-cyclodextrin (1), compound 2 was synthesized according to the method of Baer HH et al. (Carbohydr. Res. 228: 307-314). The experimental yield was 92%.
[0012] 6-[1-(4-methoxyphenyl)-1-propanol)-amino-6-deoxy-β-cyclodextrin ( 3 ) preparation
[0013] Add 1-(4-methoxyphenyl)-1-propanolamine (135 mmol) to compound 2 (0.45 mmol), stir and heat to 80 o C, reacted for 48 hours. After the reaction solution was cooled to room temperature, 5 ml of methyl tert-butyl ether was added, the precipitate was filtered and washed with 10×10 ml of acetone, and dried in vacuo to obtain compound 3 as a pale yellow solid with a yield of 91%. [R] D +12°( c = 1.0 in DMF at 25 °C); LC-ESIMS m / z [M+2] 2+ / 2, [M + 3] 3+ / 3; 1 H NMRδppm (400 MHz, CD 3 SOCD 3 ) 7.06 (d, J = 7.5Hz, 14H), 6.67 (d, J = 7.5Hz, 14H), 4.90 (bd, 7H, H-1), 3.72 (m, 7H, H-5), 3.68 (m, 7H, H-3), 3.65 (m, 7H, H-2) , 3.65 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com