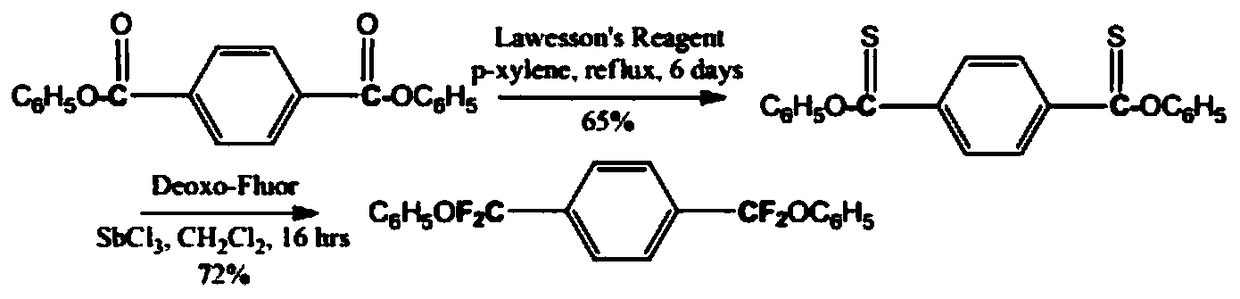
Method for synthesizing 1,4-di(difluoro phenoxy methyl) benzene
A technology of difluorophenoxymethyl and synthetic method, which is applied in the field of 1, can solve the problems of unsuitability for industrialized production, high risk of sodium hydride, high price, etc., and achieve cheap raw materials, short reaction time, and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 5.0g of 1,4-bis(difluoromethyl)benzene, 50mL of carbon tetrachloride, and 0.25g of benzoyl peroxide into the three-neck flask, replace the air, blow in chlorine gas, and stir at 80°C for 9h. After the completion of the reaction monitored by thin-layer chromatography, nitrogen gas was blown in to replace the dissolved chlorine in the reaction solution. After the gas replacement was completed, the organic phase was washed with water until neutral. Use sodium thiosulfate solution to wash the organic phase to remove benzoyl peroxide until the starch potassium iodide test paper does not change color. The organic phase was separated and washed 3 times with water. The organic phase was dried over anhydrous magnesium sulfate. Anhydrous magnesium sulfate was removed by filtration, and the solvent was distilled off under reduced pressure to obtain 9.07 g of a crude 1,4-bis(chlorodifluoromethyl)benzene product as a colorless oil.
[0030] Under nitrogen atmosphere, 9.07g of...
Embodiment 2
[0032] Add 5.0 g of 1,4-bis(difluoromethyl)benzene, 50 mL of chloroform, and 1 g of lauryl peroxide into a three-neck flask, replace the air with chlorine gas, and stir at 80°C for 9 h. After the completion of the reaction monitored by thin-layer chromatography, nitrogen gas was blown in to replace the dissolved chlorine in the reaction solution. After the gas replacement was completed, the organic phase was washed with water until neutral. Use sodium thiosulfate solution to wash the organic phase to remove benzoyl peroxide until the starch potassium iodide test paper does not change color. The organic phase was separated and washed 3 times with water. The organic phase was dried over anhydrous magnesium sulfate. Anhydrous magnesium sulfate was removed by filtration, and the solvent was distilled off under reduced pressure to obtain 9.35 g of crude product of 1,4-bis(chlorodifluoromethyl)benzene as a colorless oil.
[0033] Under nitrogen atmosphere, 9.35g of the above crud...
Embodiment 3
[0035] Add 5.0g of 1,4-bis(difluoromethyl)benzene, 50mL of trichlorobenzene, and 0.5g of tert-butyl peroxyvaleric acid to a three-neck flask, replace the air with chlorine gas, and stir at 80°C for 9h . After the completion of the reaction monitored by thin-layer chromatography, nitrogen gas was blown in to replace the dissolved chlorine in the reaction solution. After the gas replacement was completed, the organic phase was washed with water until neutral. Use sodium thiosulfate solution to wash the organic phase to remove benzoyl peroxide until the starch potassium iodide test paper does not change color. The organic phase was separated and washed 3 times with water. The organic phase was dried over anhydrous magnesium sulfate. Anhydrous magnesium sulfate was removed by filtration, and the solvent was distilled off under reduced pressure to obtain 8.98 g of a crude 1,4-bis(chlorodifluoromethyl)benzene product as a colorless oil.
[0036]Under a nitrogen atmosphere, 8.98g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com