Liquid crystal monomer fluorine dioxane contained compound and synthesis method thereof
A technology containing fluorooxane and liquid crystal monomer, applied in organic chemistry and other directions, can solve problems such as low rotational viscosity, and achieve the effect of increasing molecular width and simple and easy synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
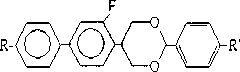
[0016] Embodiment 1, a liquid crystal monomer containing fluorooxane compound, the structure of the compound is as follows:
[0017] , where R is one of fluorine, chlorine, H, C1-C5 straight-chain alkyl, C1-C5 straight-chain alkoxy, R' is fluorine, chlorine, H, C1-C5 straight-chain alkyl, One of C1-C5 linear alkoxy groups and cyano groups, R and R' are the same or different. This type of compound can be used alone or in combination for liquid crystal displays in various display forms. The following synthesis route is adopted:
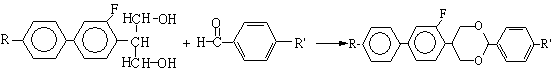
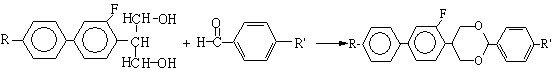
[0018]
[0019] I II III
[0020] 2-(2'-fluoro-4'-p-alkylphenyl) phenylpropanediol with the structure shown in I and p-alkylbenzaldehyde with the structure shown in II are used as raw materials, wherein R in compounds I and II , R' are the same as R and R' in the fluorooxane compound III, using p-toluenesulfonic acid as a catalyst, using an organic solvent as a reaction medium, and reacting for 2-12 hours at a temperature ...
Embodiment 2
[0023] Example 2, a liquid crystal monomer containing fluorine-containing oxane compound and its synthesis method. The liquid crystal monomer containing fluorine-containing oxane compound in this example is specifically 5-(2'-fluoro-4'-p-n-propylbenzene Base) phenyl-2-(p-ethylphenyl)-1,3-dioxane, represented by IIIb;
[0024] Add 29 g of 2-(2'-fluoro-4'-p-n-propylphenyl) phenylpropanediol and 14 g of p-ethylbenzaldehyde into a 250 mL three-necked reaction flask equipped with mechanical stirring, reflux condenser, and addition funnel. 3g of p-toluenesulfonic acid, 110mL of toluene, started stirring and heating, 108-110°C reflux reaction for 3-8h, other conditions were the same as in Example 1, and purified to obtain 29g of product IIIb, ωⅢb: 99.5%, yield: 71.4%. Product mass spectrometry results: ES-MS m / z: 404[M]+.
[0025] The present invention selects biphenyl and oxane as the molecular skeleton, introduces fluorine substituents on the side of biphenyl, designs fluorine-con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com