Transdermally absorbed preparation of anti-dementia drug
A preparation and drug technology, applied in the field of percutaneous absorption preparations, can solve the problems of low drug permeability and difficult absorption, and achieve the effect of inhibiting skin irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
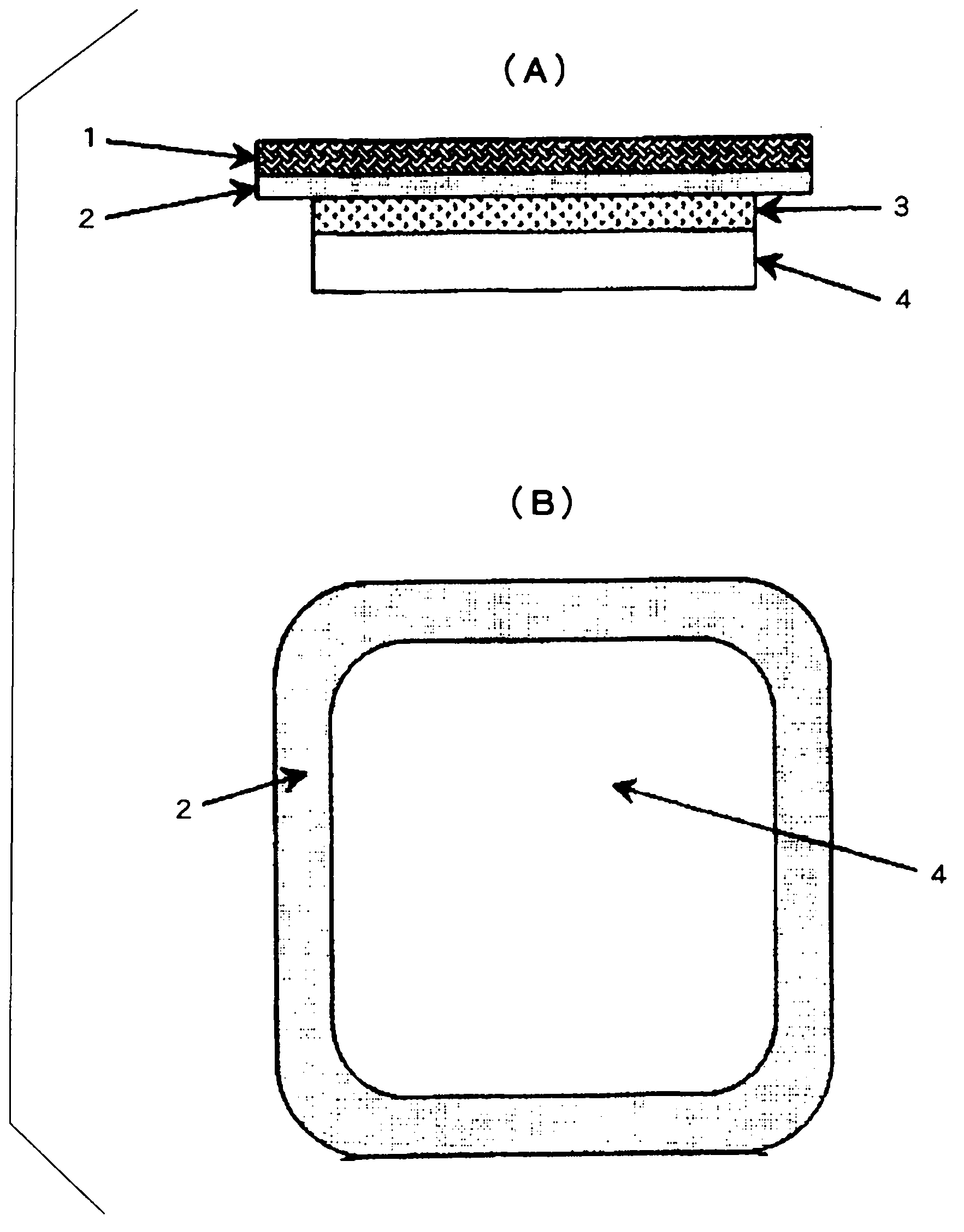
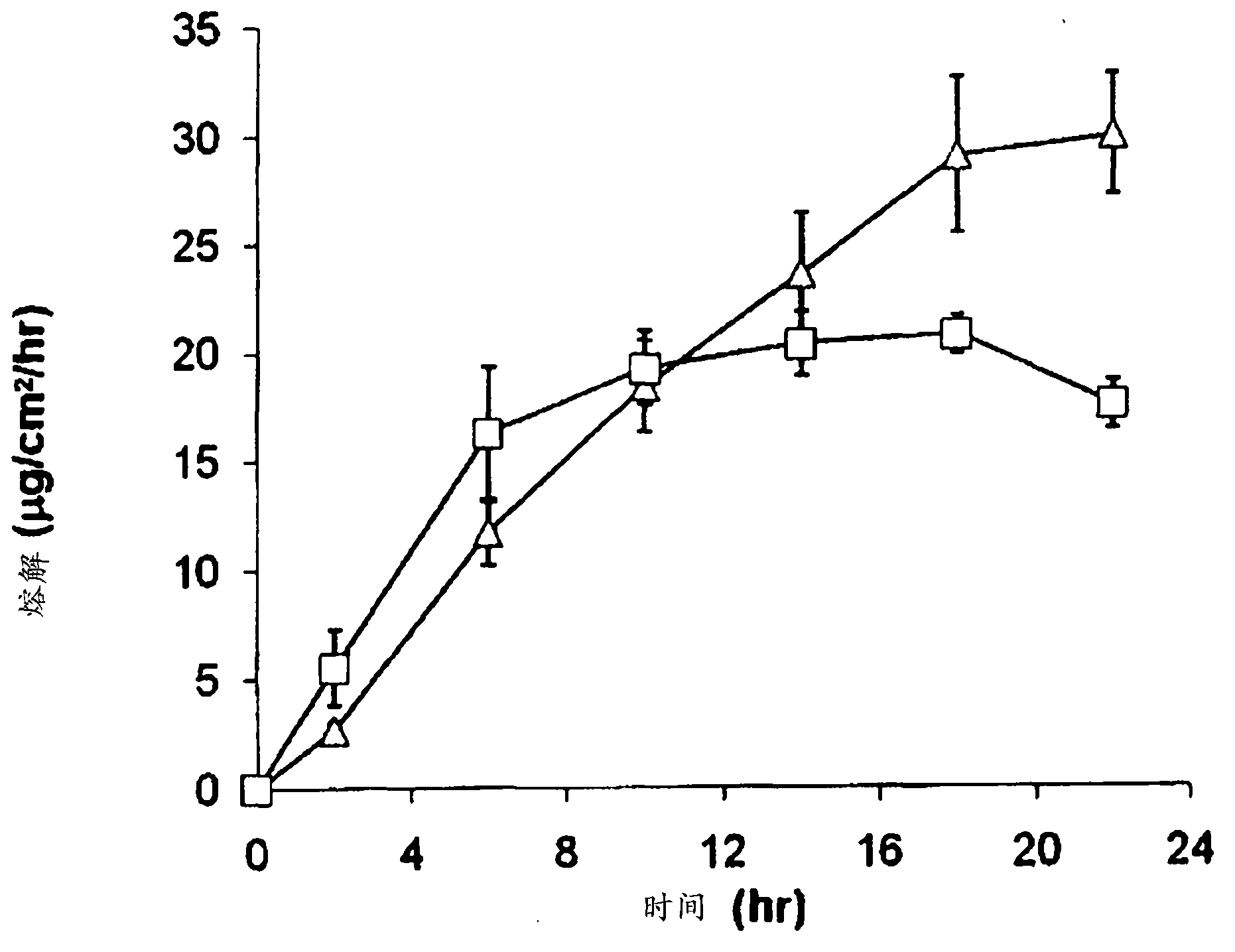
Image
Examples
Embodiment 1
[0085] Example 1: Drug-containing layer containing 12.5% by mass of donepezil hydrochloride Preparation of drug-containing layer
[0086] Prepare Donepezil Hydrochloride, Eudragit(注册商标) E 100, triethyl citrate, glycerin, and SML (sorbitan monolaurate) were mixed in an appropriate amount of toluene and stirred. SIS (styrene-isoprene-styrene block copolymer, Kraton (注册商标) D1111K, manufactured by Kraton Corporation) to obtain a paste solution.
[0087] [Table 1]
[0088]
[0089] The said paste solution was apply|coated to the liner made of polyethylene terephthalate, and it dried at 80 degreeC for 15 minutes, and obtained the drug-containing layer. Adjusted so that the amount of the drug-containing layer after drying is 50 g / m 2 .
[0090] Then, a support layer (Scotchpak (商标) 9732, manufactured by 3M). After that, the liner was peeled off from the drug-containing layer to obtain a laminate.
[0091] Setting of the fixing mechanism
[0092] Put Duro-Tak (商标) ...
Embodiment 2
[0094] Example 2: Drug-containing layer containing 6.25% by mass of donepezil hydrochloride
[0095] A percutaneous absorption preparation was prepared in the same manner as in Example 1, except that the amount of donepezil hydrochloride in the drug-containing layer was changed to 6.25% by mass in Example 1. In addition, the amounts of other components other than donepezil hydrochloride were not changed compared with Example 1, but the following formulations were obtained when the ratios of the components were expressed based on the drug-containing layer.
[0096] [Table 2]
[0097]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com