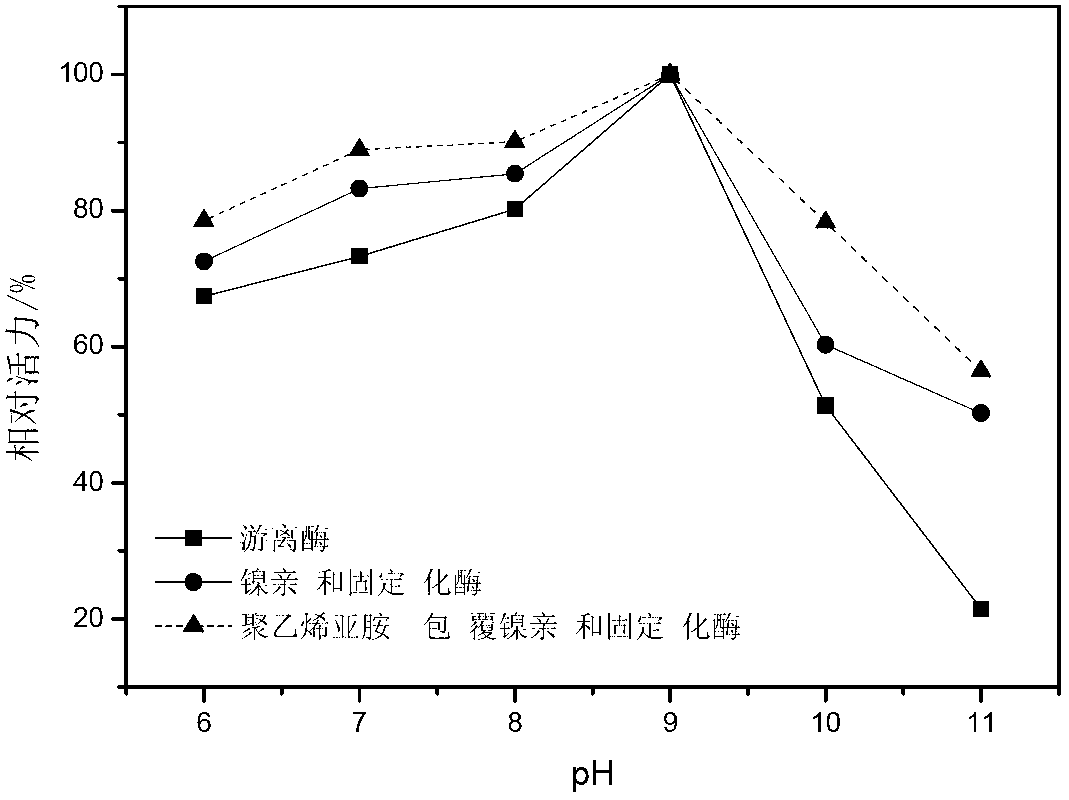
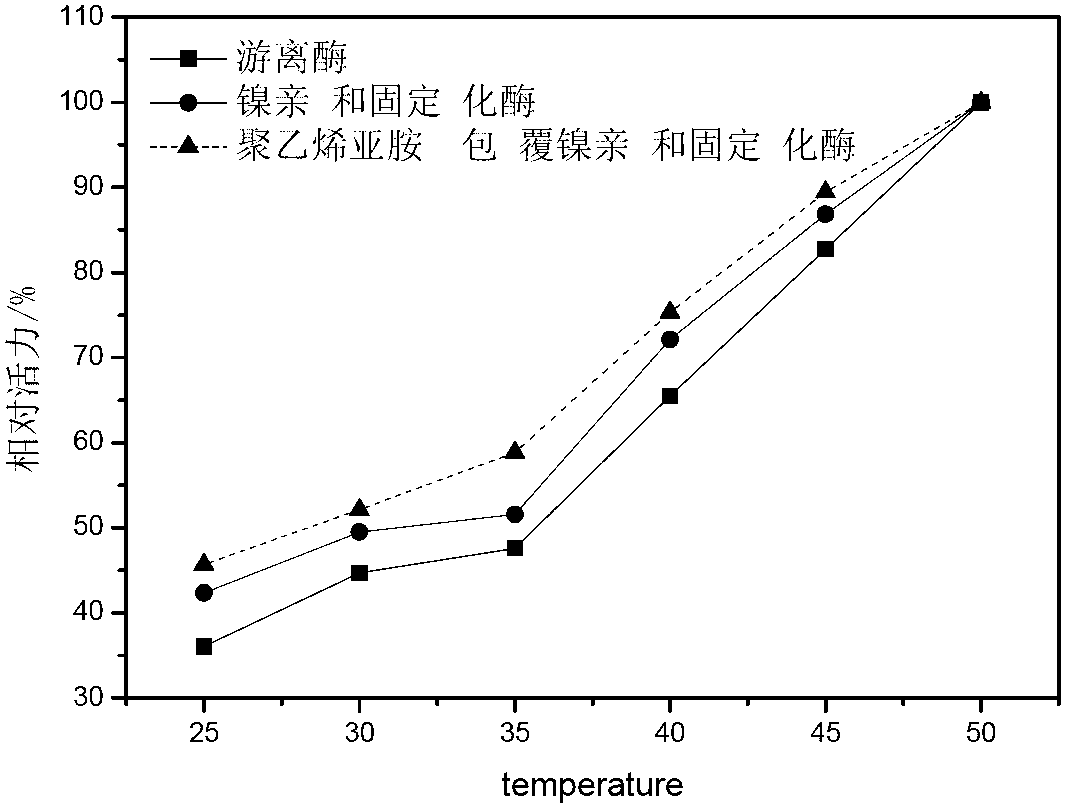
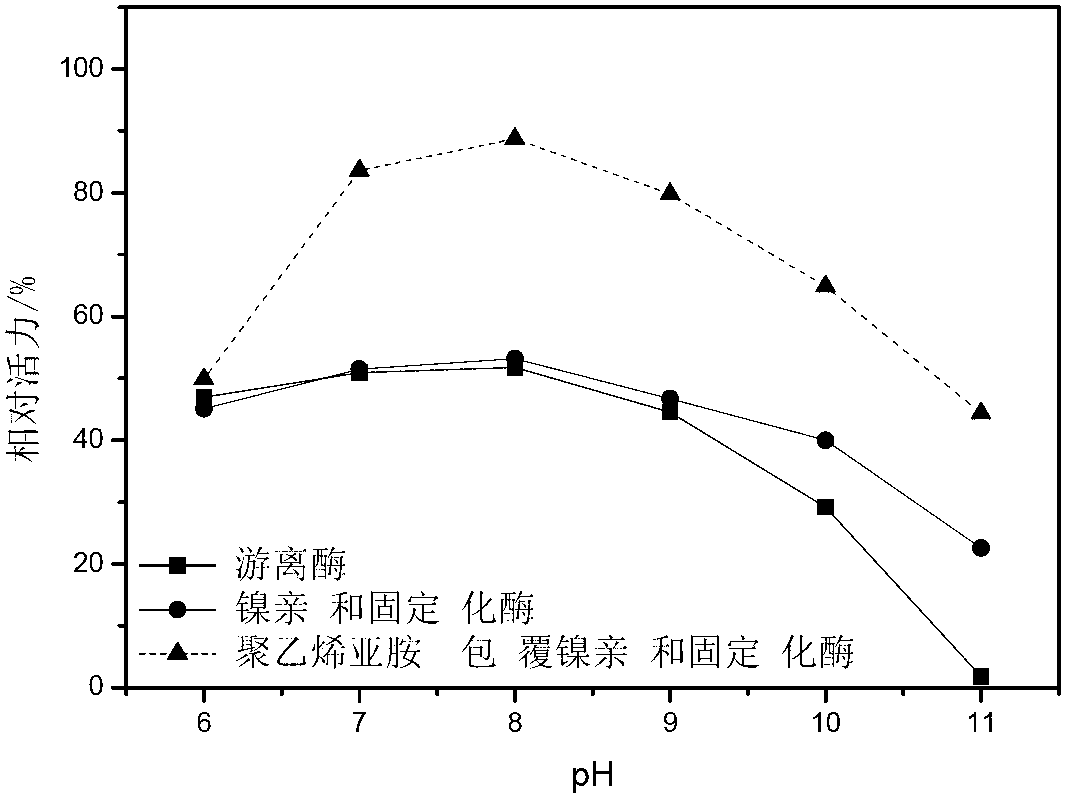
Gamma-glutamyltranspeptidase immobilized enzyme, preparation method and application thereof
A technology of glutamyl transpeptidase and immobilized enzyme, which is applied in the fields of biotechnology and enzyme engineering, can solve the problems of limited pH stability, irreversible decline in enzyme activity, limited application, etc., and achieves good reuse performance, pH Stability-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Immobilization of recombinant γ-glutamyl transpeptidase on Ni Sepharose 6 Fast Flow affinity carrier: Weigh 1.0g Ni Sepharose 6 Fast Flow affinity carrier and place it in a sand core tube, add recombinant γ-glutamyl transpeptidase Enzyme cell disruption solution 10mL (enzyme activity: 13.06U / mL), shake reaction in ice bath at 4°C for 1h. After the reaction, wash off excess enzyme solution and impurities twice with pH 8.0 50mmol / L Tris-HCl buffer solution containing 200mmol / L imidazole, and then wash the carrier twice with pH 8.050mmol / L Tris-HCl buffer solution , that is, the nickel affinity carrier immobilized recombinant gamma-glutamyl transpeptidase was prepared. Its enzyme activity is 26.1U / g carrier, and the recovery rate of enzyme activity is 20.0%. Add the obtained nickel affinity carrier-immobilized recombinant γ-glutamyl transpeptidase into 0.2% polyethyleneimine aqueous solution at pH 7.0, shake at 4°C for 12 hours, and buffer with Tris-HCl at pH 8.050mmol / L ...
Embodiment 2
[0031] Add 1.0g Ni Sepharose 6Fast Flow to 10mL of cell disruption solution containing recombinant γ-glutamyl transpeptidase (enzyme activity is 13.06U / mL), shake and react at 45°C for 6h, then filter out the liquid; The particles were repeatedly washed twice with 30mmol / L Tris-HCl buffer containing 200mmol / L imidazole at pH 7.0, and the liquid was filtered off; the solid particles were washed twice with 30mmol / L Tris-HCl buffer at pH 7.0 , the liquid was filtered out; the solid particles were then washed with 10 mL of 0.2% polyethyleneimine aqueous solution at pH 7.0, shaken and reacted at 4°C for 12 hours, and then washed repeatedly with 30 mmol / L Tris-HCl buffer solution of pH 7.0 for 2 The second time, the liquid is filtered out to obtain the γ-glutamyl transpeptidase immobilized enzyme.
Embodiment 3
[0033]Add 1.0g Ni Sepharose 6Fast Flow to 30mL of cell disruption solution containing recombinant γ-glutamyl transpeptidase (enzyme activity is 13.06U / mL), shake and react at 45°C for 6h, then filter out the liquid; The particles were repeatedly washed 3 times with 60mmol / L Tris-HCl buffer containing 200mmol / L imidazole at pH 9.0, and the liquid was filtered off; the solid particles were washed 3 times with 60mmol / L Tris-HCl buffer at pH 9.0 , the liquid was filtered out; the solid particles were then washed with 30mL of 1% polyethyleneimine aqueous solution with pH 9.0 and shaken at 45°C for 24 hours, and then the solid particles were washed repeatedly with 60mmol / L Tris-HCl buffer solution of pH 9.0 3 times, the liquid was filtered out to obtain γ-glutamyl transpeptidase immobilized enzyme.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com