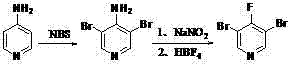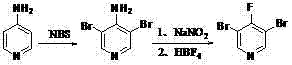Synthesis of 3, 5-dibromo-4-iodopyridine
A kind of technology of fluoropyridine and aminopyridine is applied in the synthesis field of halopyridine, and achieves the effect of good economic benefit and social benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of 3,5-dibromo-4-aminopyridine (bromination reaction): Add 400ml of solvent carbon tetrachloride, 47.05g (0.5mol) of 4-aminopyridine, azobisisobutyl Nitrile etc. (AIBN) 0.41g, add N-bromosuccinimide (NBS) 195.78g (1.1mol) in batches at 20°C, then raise the temperature to 50°C for 4 hours, TLC board monitors the raw material 4-amino Pyridine and the intermediate 3-bromo-4-aminopyridine are converted into the target product 3,5-dibromo-4-aminopyridine, and the reaction is stopped. Post-reaction treatment: the reaction solution was stirred and cooled to room temperature, filtered, the filter cake was fully washed with carbon tetrachloride several times, the filtrate was washed once with aqueous sodium bicarbonate solution, once with saturated saline, and the solvent carbon tetrachloride was removed by rotary evaporation , the obtained 3,5-dibromo-4-aminopyridine crude product was 120.60 g, and the crude product was directly used in the next reaction.
[0022] Sy...
Embodiment 2
[0024] The bromination reagent was 314.51 g (1.1 mol) of 1,3-dibromo-5,5-dimethylhydantoin, and other operations were the same as in Example 1.
[0025] The operation was carried out according to Example 1, and 97.0 g of pure white 3,5-dibromo-4-fluoropyridine was obtained, with a content of 98.6% and a yield of 76.1%.
Embodiment 3
[0027] Described bromination reaction initiator is 0.61g of benzoyl peroxide, and other are the same as operation embodiment 1.
[0028] The operation was carried out according to Example 1, and 102.2 g of pure white 3,5-dibromo-4-fluoropyridine was obtained, with a content of 99.0% and a yield of 80.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com