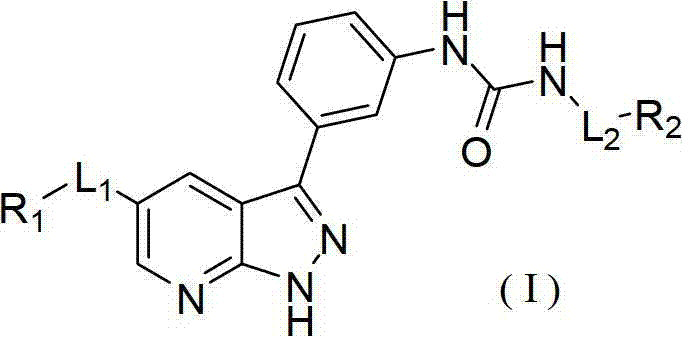
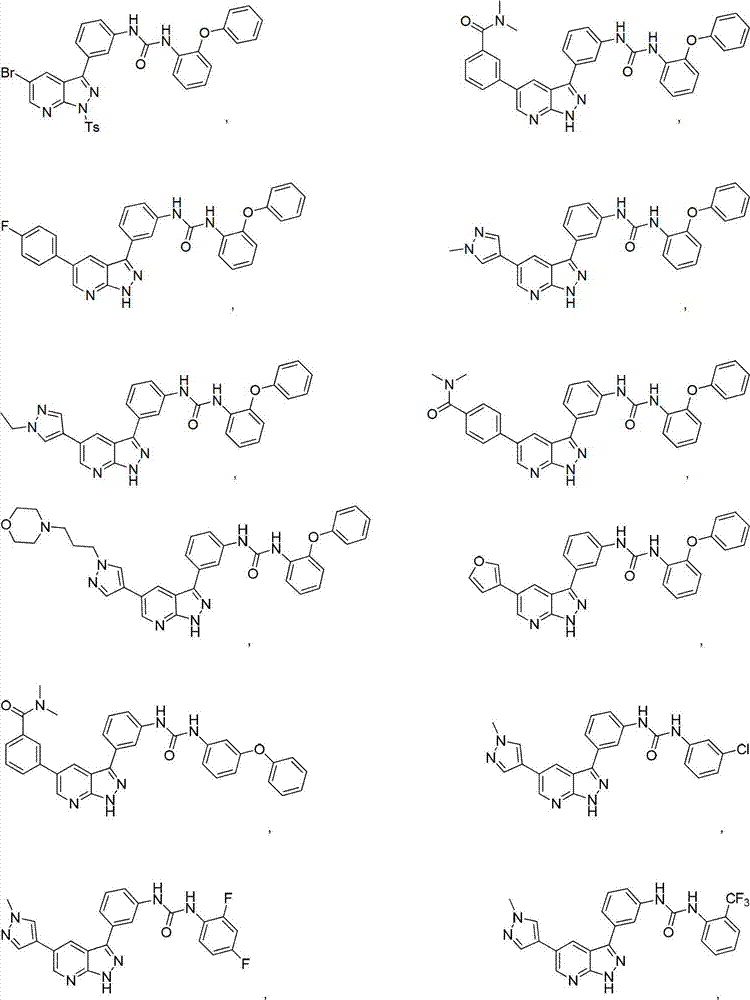
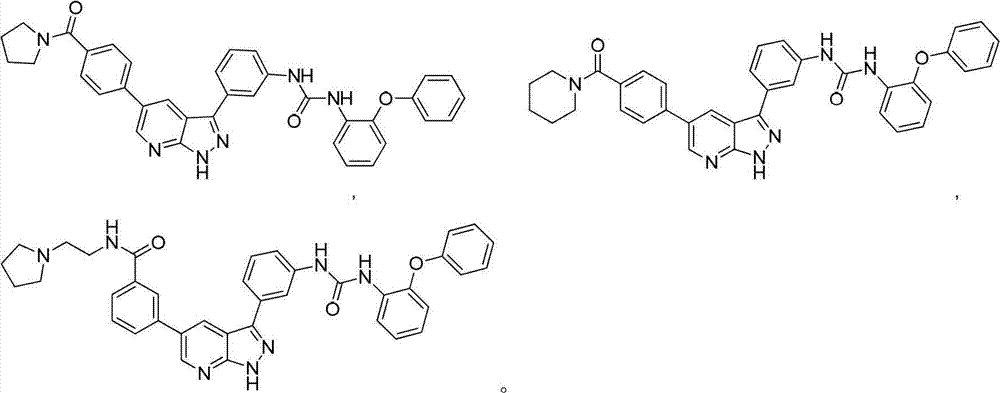
Pyrazolopyridine derivatives for inhibiting activity of insulin-like growth factor-1 receptor (IGF-1R) tyrosine kinase
A C5-C10, C6-C10 technology, applied in the field of pyridopyrazole derivatives that inhibit IGF-1R tyrosine kinase activity, can solve the problems of weakened ability to inhibit IGF-1R, lack of transcriptional activity of BRCAI, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: 1-(3-(5-bromo-1-p-toluenesulfonyl-1H-pyrazol[3,4-b]pyridin-3-yl)phenyl)-3-(2-phenoxy Phenyl)urea (I-c-1)
[0085]
[0086] step 1
[0087] 2.00g (6.17mmol) of 5-bromo-3-iodo-1H-pyrazol[3,4-b]pyridine was dissolved in 25mL of dichloromethane, and 1.41g (7.4mmol) of p-toluenesulfonyl chloride and 1g of 50% NaOH aqueous solution were added , then add 10mL water, 0.1g (0.31mmol) tetrabutylammonium bromide, react at room temperature for 24 hours, pour into water, separate the liquids, extract the water phase with dichloromethane once, combine the organic phases, add anhydrous Dry over sodium sulfate, filter, add 100~200 mesh silica gel to the filtrate to make sand, perform column chromatography, and elute with petroleum ether: ethyl acetate = 1:1 to obtain 2.55g (86%) of 5-bromo-3-iodo-1 - p-toluenesulfonyl-1H-pyrazol[3,4-b]pyridine (I-a) as a white solid. 1 H NMR (400MHz, DMSO-d 6 )δ8.89(d,1H,-ArH),8.37(d,1H,-ArH),7.94(d,2H,2×-ArH),7.46(d,2H,2×-ArH),2.36(s...
Embodiment 2
[0097] Example 2: N,N-Dimethyl-3-(3-(3-(3-(2-phenoxyphenyl)ureido)phenyl)-1H-pyrazol[3,4-b] Pyridin-5-yl)benzamide (I-1)
[0098]
[0099] step 1
[0100] To 30mg (0.045mmol) 1-(3-(5-bromo-1-p-toluenesulfonyl-1H-pyrazol[3,4-b]pyridin-3-yl)phenyl)-3-(2-benzene Oxyphenyl) urea (I-c-1), 10 mg (0.052 mmol) 3-(dimethylcarbamoyl) phenylboronic acid, 3 mg (4.27 μmol) bis(triphenylphosphine) palladium dichloride in a mixture of 5 ml DMF (N,N-dimethylformamide, the same below), 0.1ml 2M.Na 2 CO 3 Aqueous solution, react at 120°C for 0.5 hours, concentrate to remove DMF, add appropriate amount of water to the residue, extract with dichloromethane, dry over anhydrous sodium sulfate, filter, add 100~200 mesh silica gel to the filtrate, concentrate to make sand, column chromatography, and dichloromethane Methane:methanol=100:1 eluted to give 30 mg (91%) N,N-dimethyl-3-(3-(3-(3-(2-phenoxyphenyl)ureido)phenyl)- 1-p-Toluenesulfonyl-1H-pyrazol[3,4-b]pyridin-5-yl)benzamide (I-d-1), as a...
Embodiment 3
[0106] Example 3: 1-(3-(5-(4-fluorophenyl)-1H-pyrazol[3,4-b]pyridin-3-yl)phenyl)-3-(2-phenoxybenzene base) urea (I-2)
[0107]
[0108] step 1
[0109]Using the method of Example 3, step 1, 30 mg (0.045 mmol) 1-(3-(5-bromo-1-p-toluenesulfonyl-1H-pyrazol[3,4-b]pyridin-3-yl)benzene Base)-3-(2-phenoxyphenyl)urea (I-c-1) reacted with 8mg (0.055mmol) 4-fluorophenylboronic acid to give 18mg (60%) of 1-(3-(5-(4- Fluorophenyl)-1-p-toluenesulfonyl-1H-pyrazol[3,4-b]pyridin-3-yl)phenyl)-3-(2-phenoxyphenyl)urea (I-d-2) , as a white solid.
[0110] step 2
[0111] Using the method of Example 3, Step 2, 18 mg (0.027 mmol) 1-(3-(5-(4-fluorophenyl)-1-p-toluenesulfonyl-1H-pyrazol[3,4-b]pyridine -3-yl)phenyl)-3-(2-phenoxyphenyl)urea (I-d-2) was reacted to give 11 mg (79%) of the title compound (I-2) as a white solid.
[0112] 1 H NMR (500MHz, DMSO-d 6 )δ13.88(s,1H,-NH-),9.44(s,1H,-NH-),8.87(s,1H,-ArH),8.71(s,1H,-ArH),8.48(s,1H ,-NH-),8.34(d,1H,-ArH),8.28(s,1H,-ArH),7.89(t,2H,2×-ArH)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com