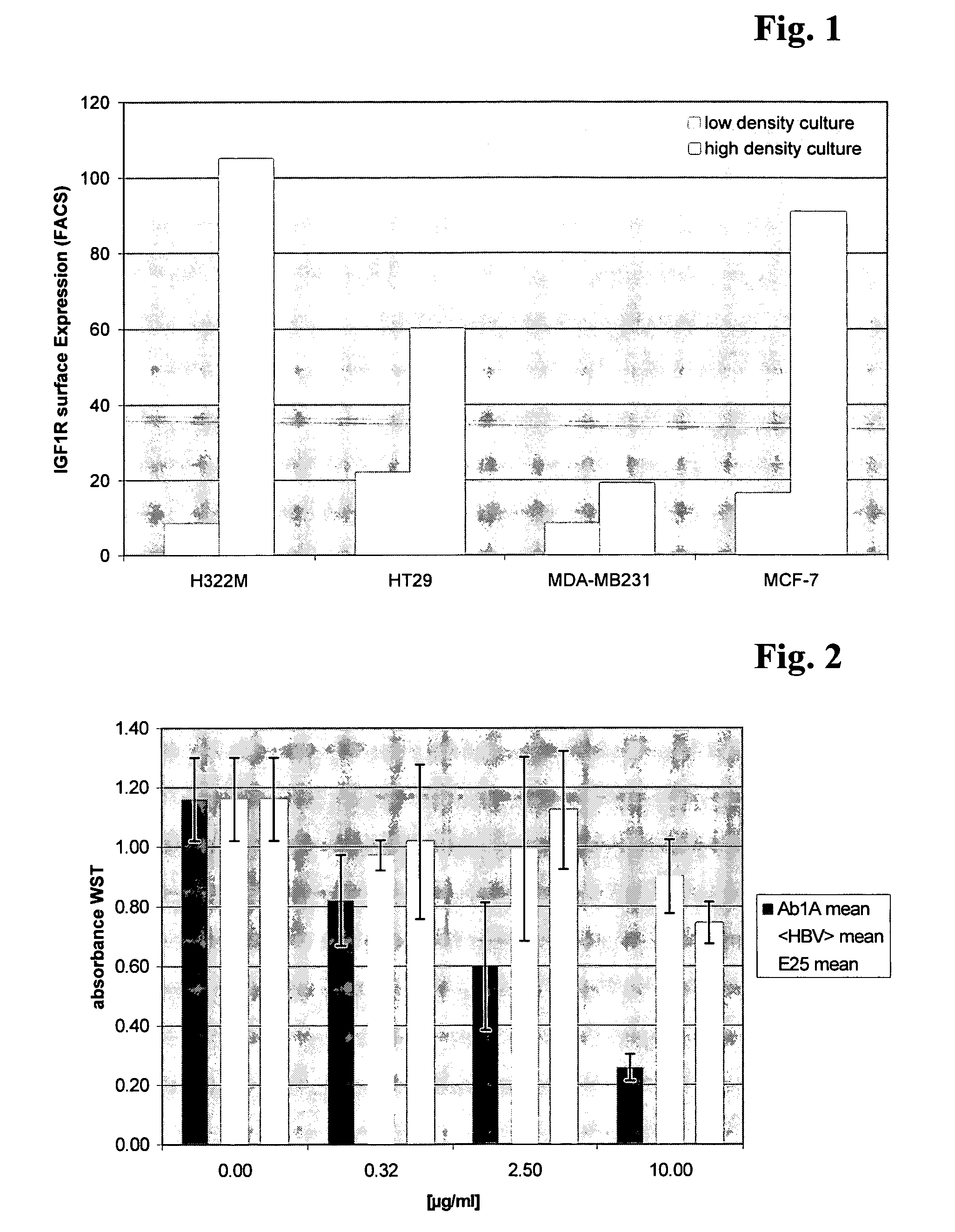
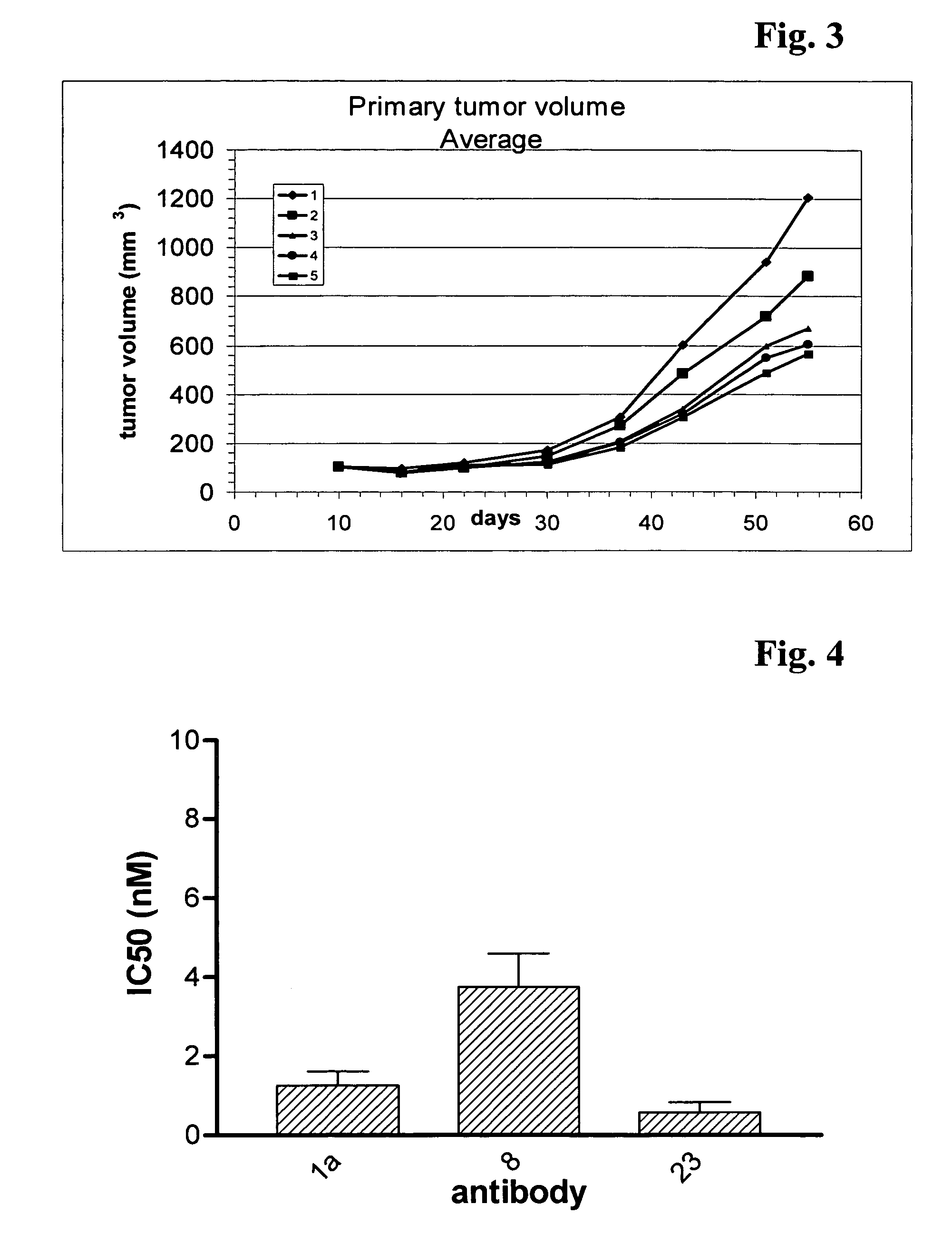
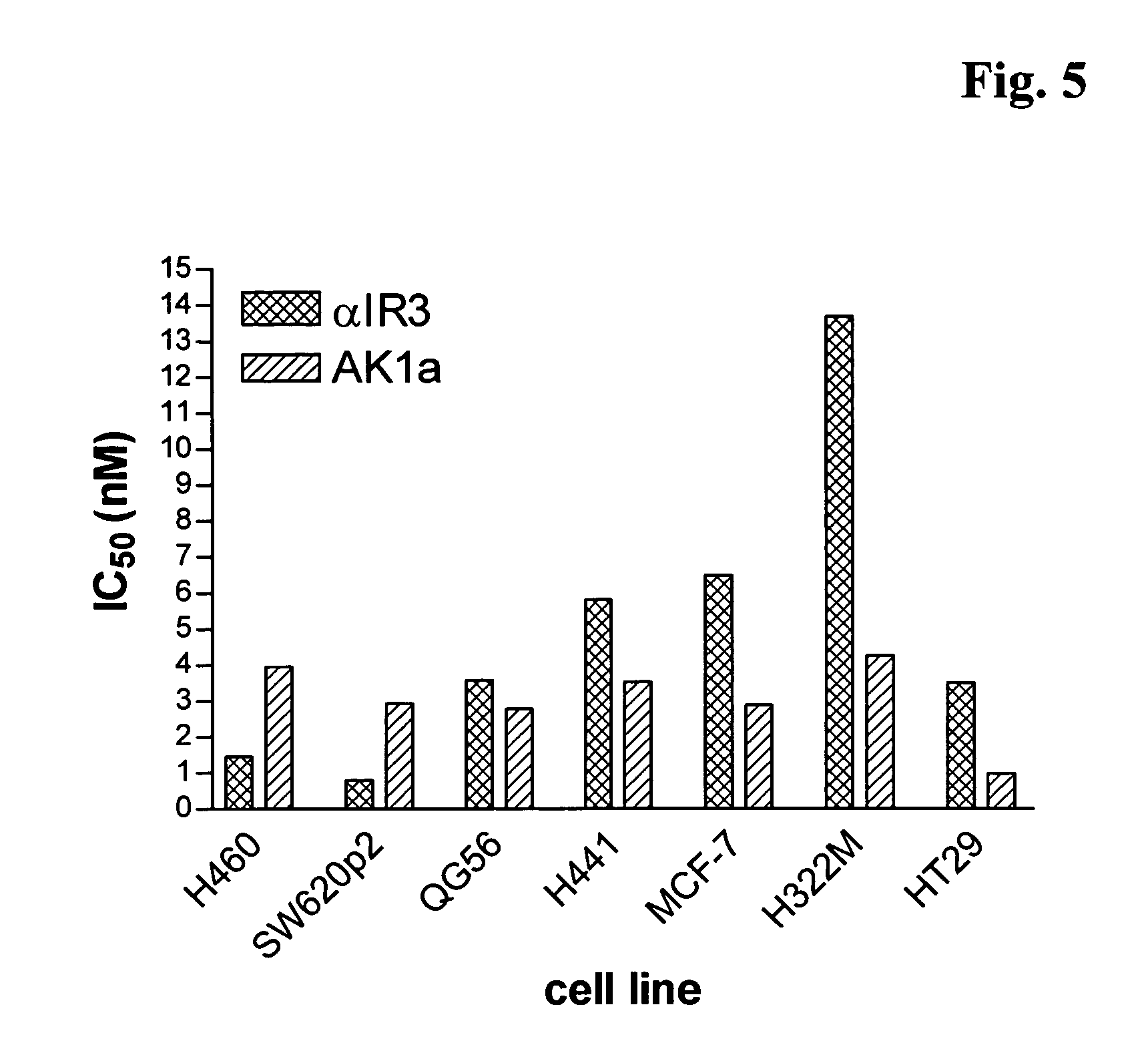
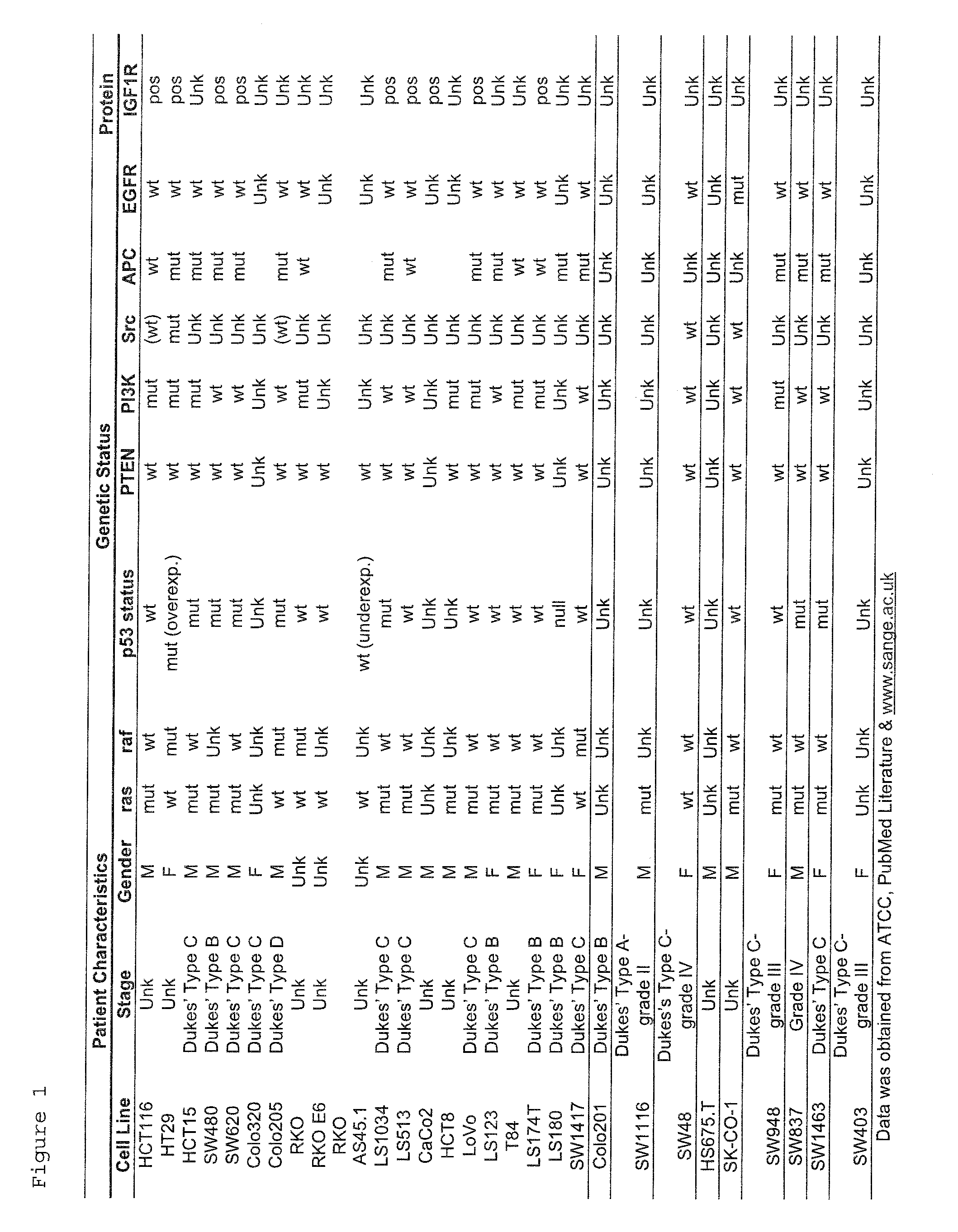
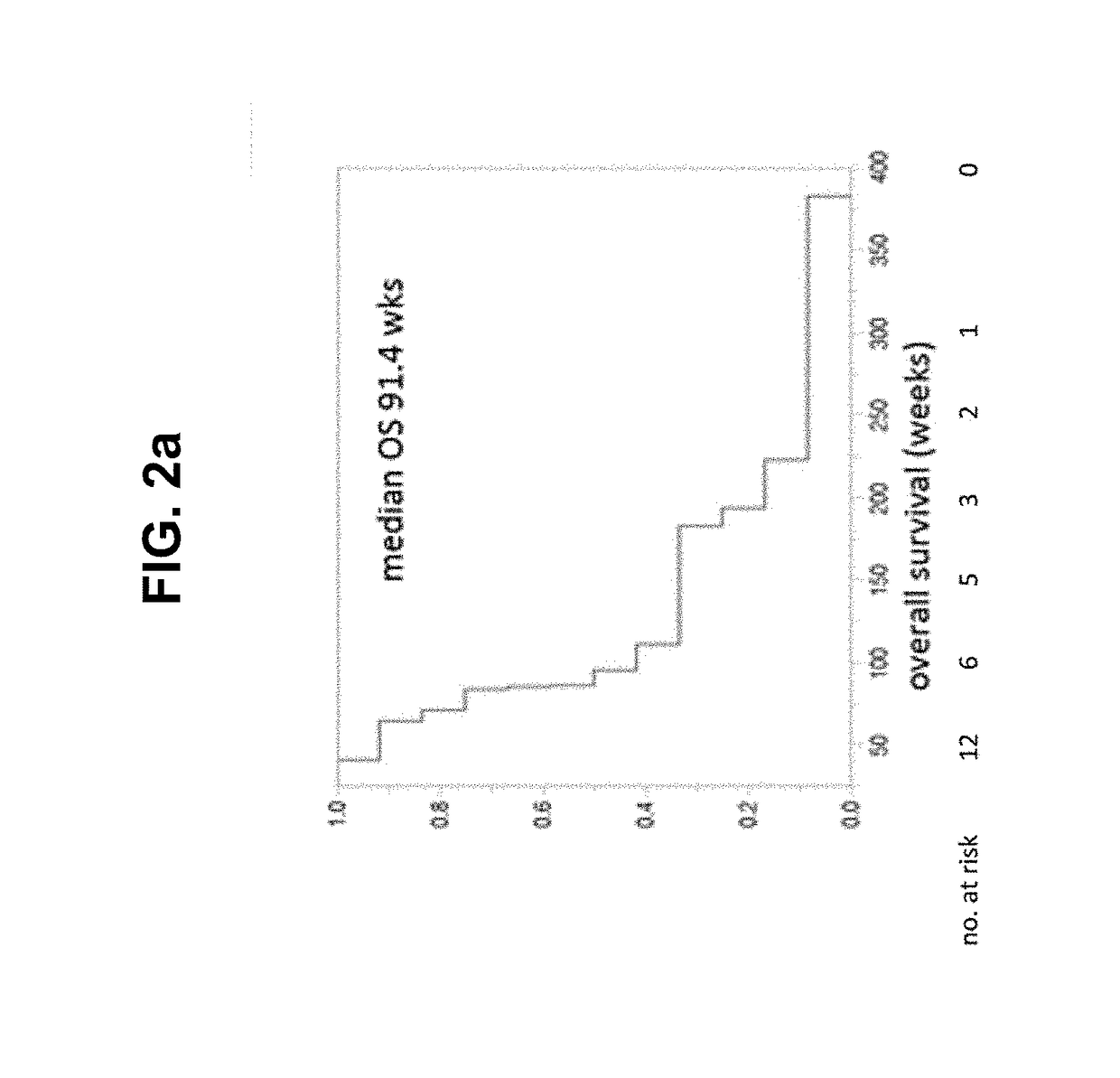
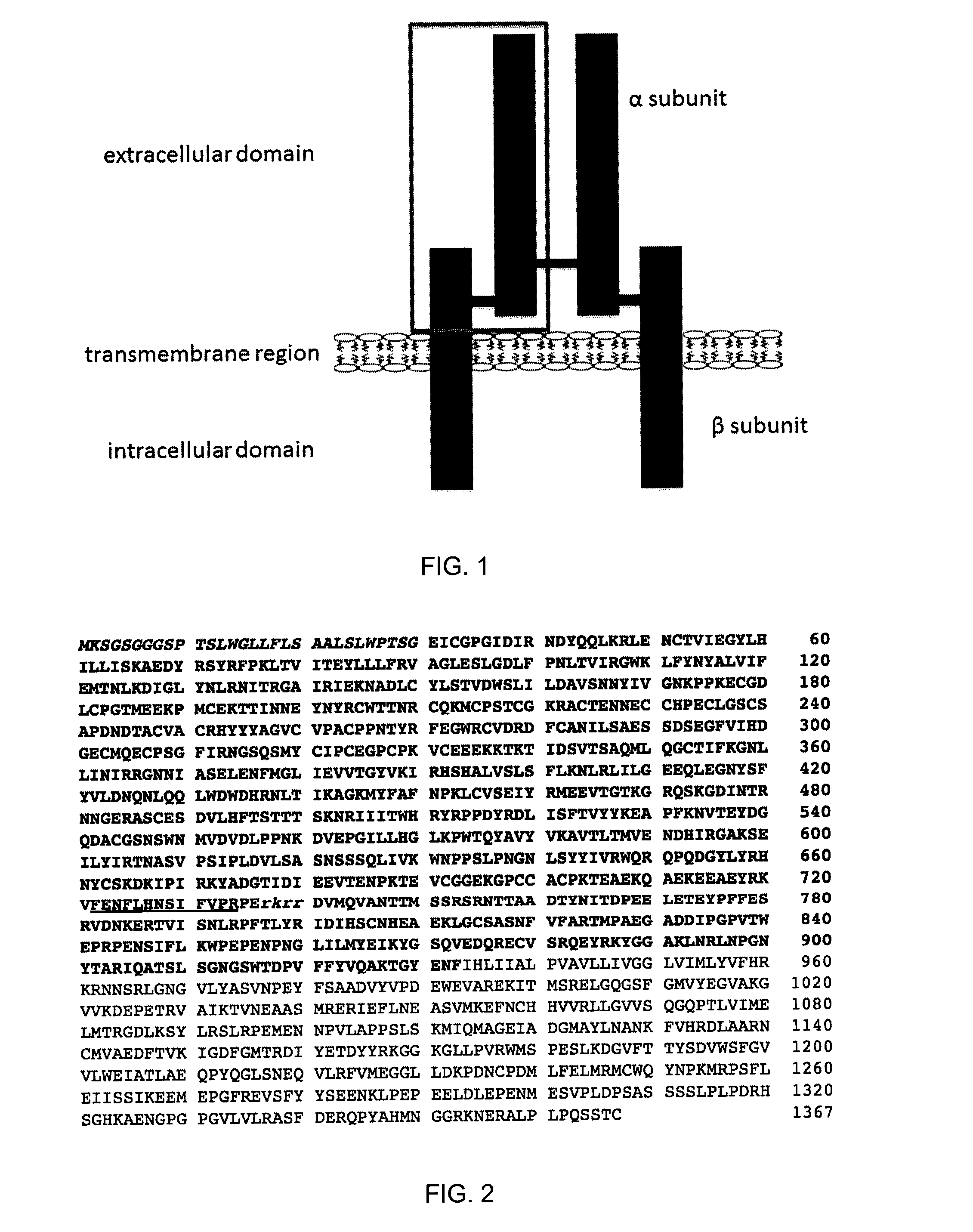
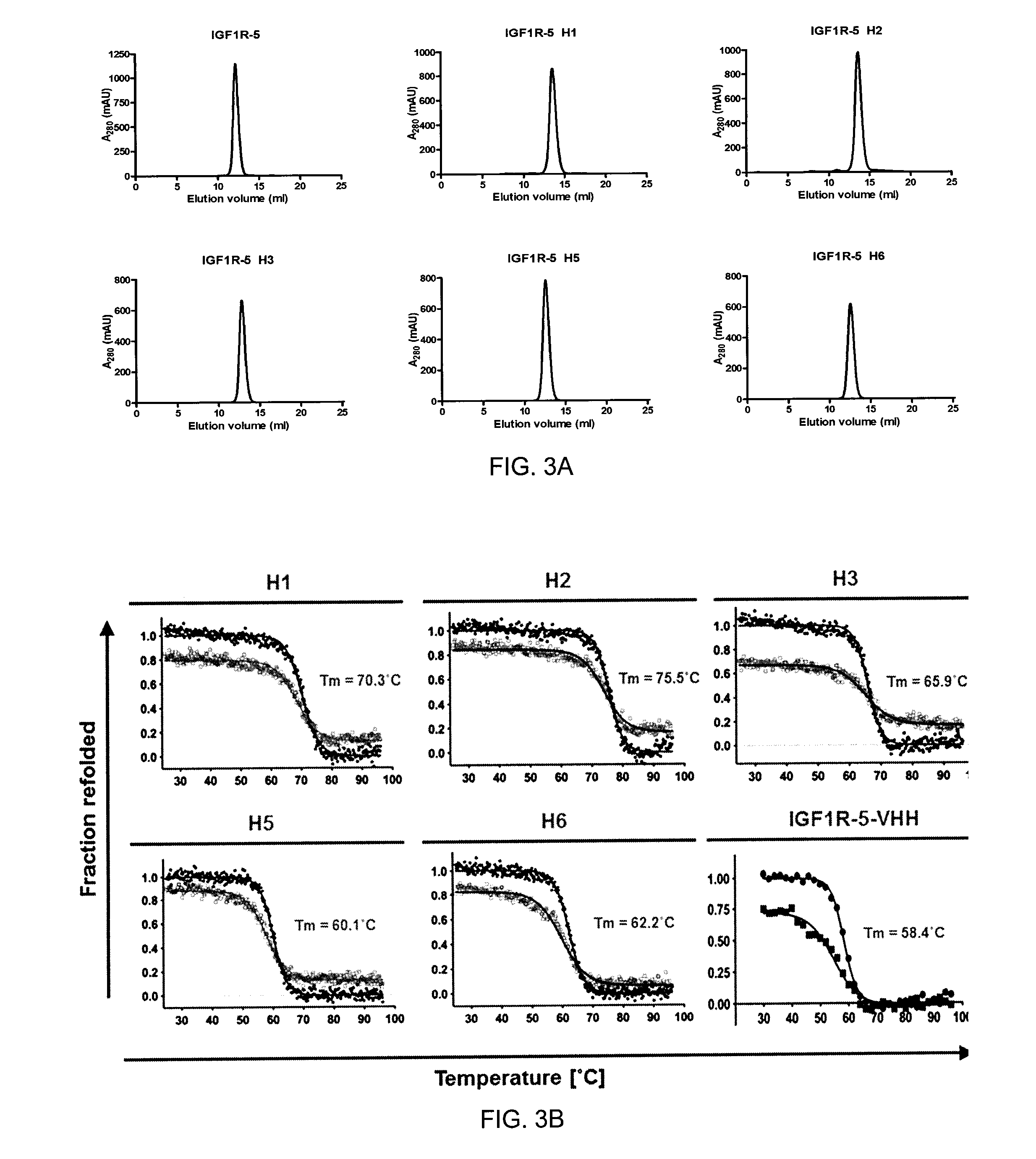
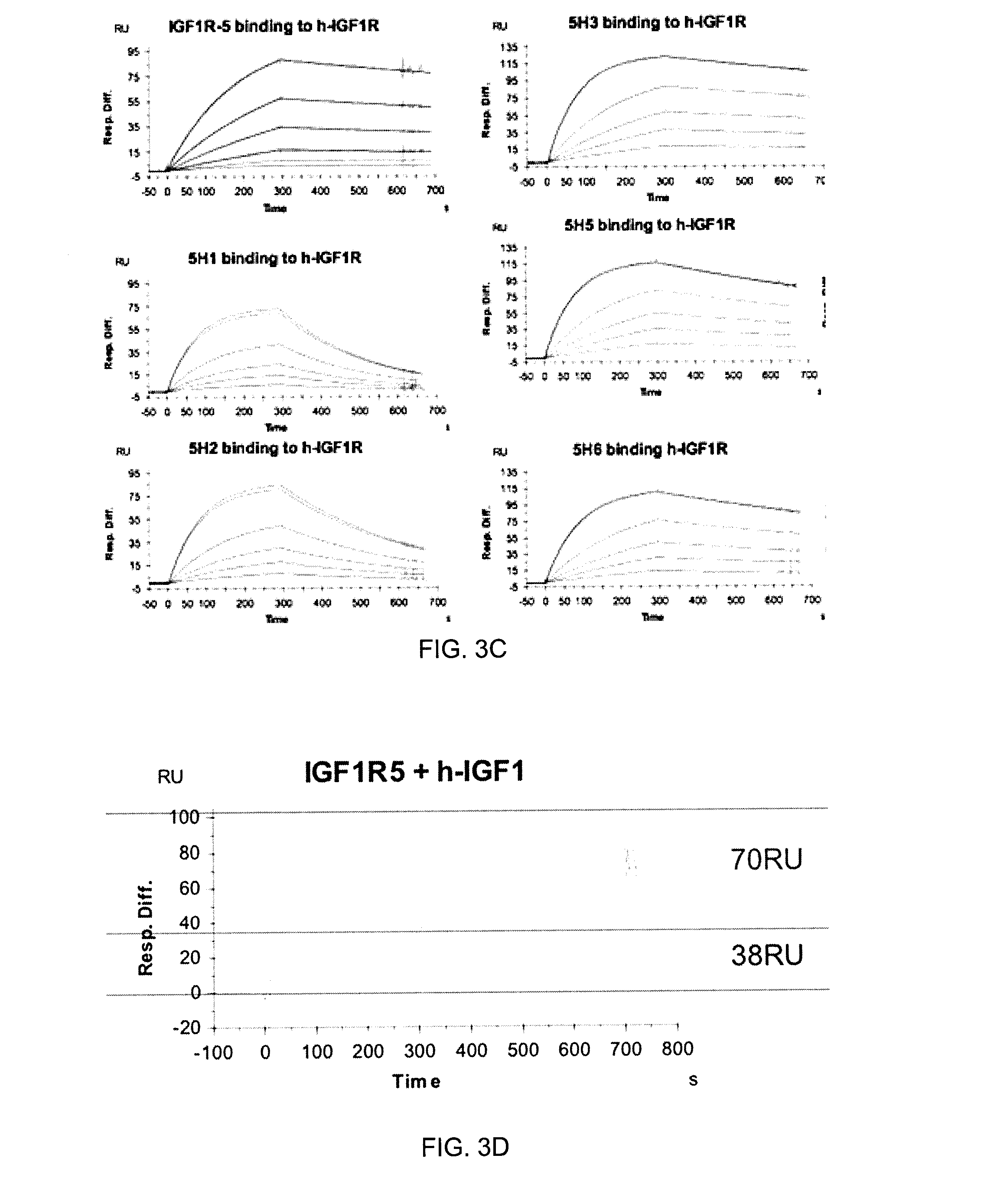
Patents
Literature
31 results about "Insulin-like growth factor 1 receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
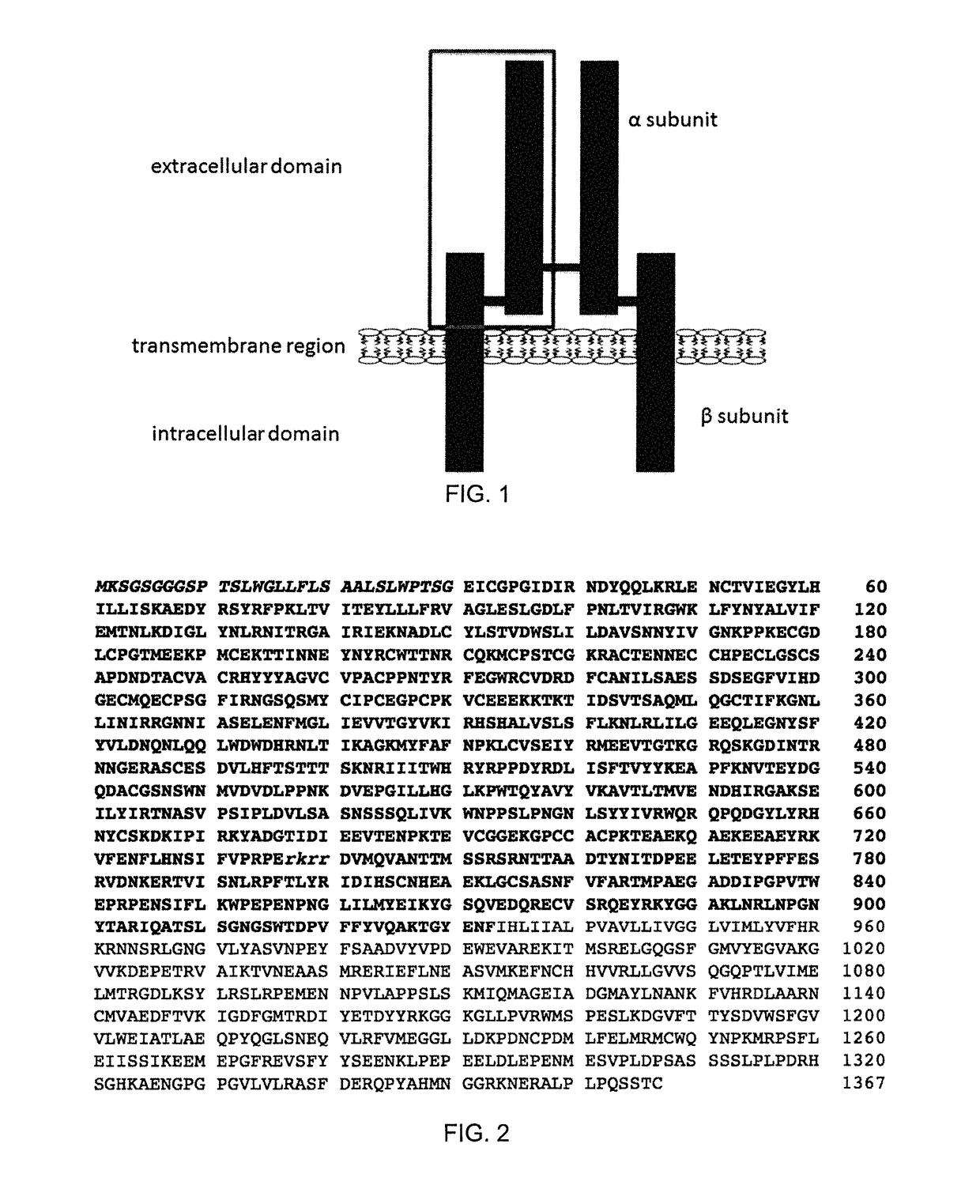
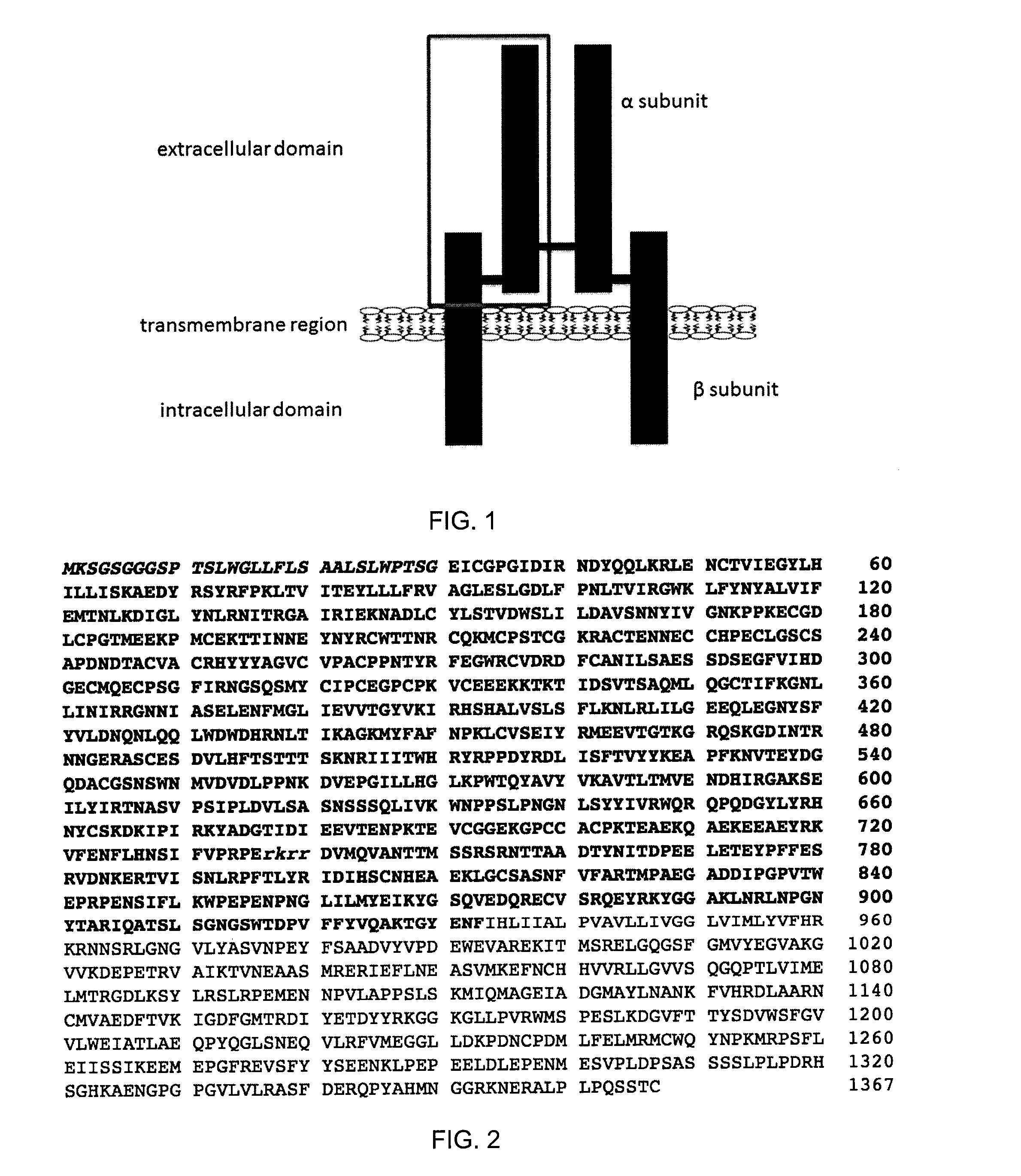
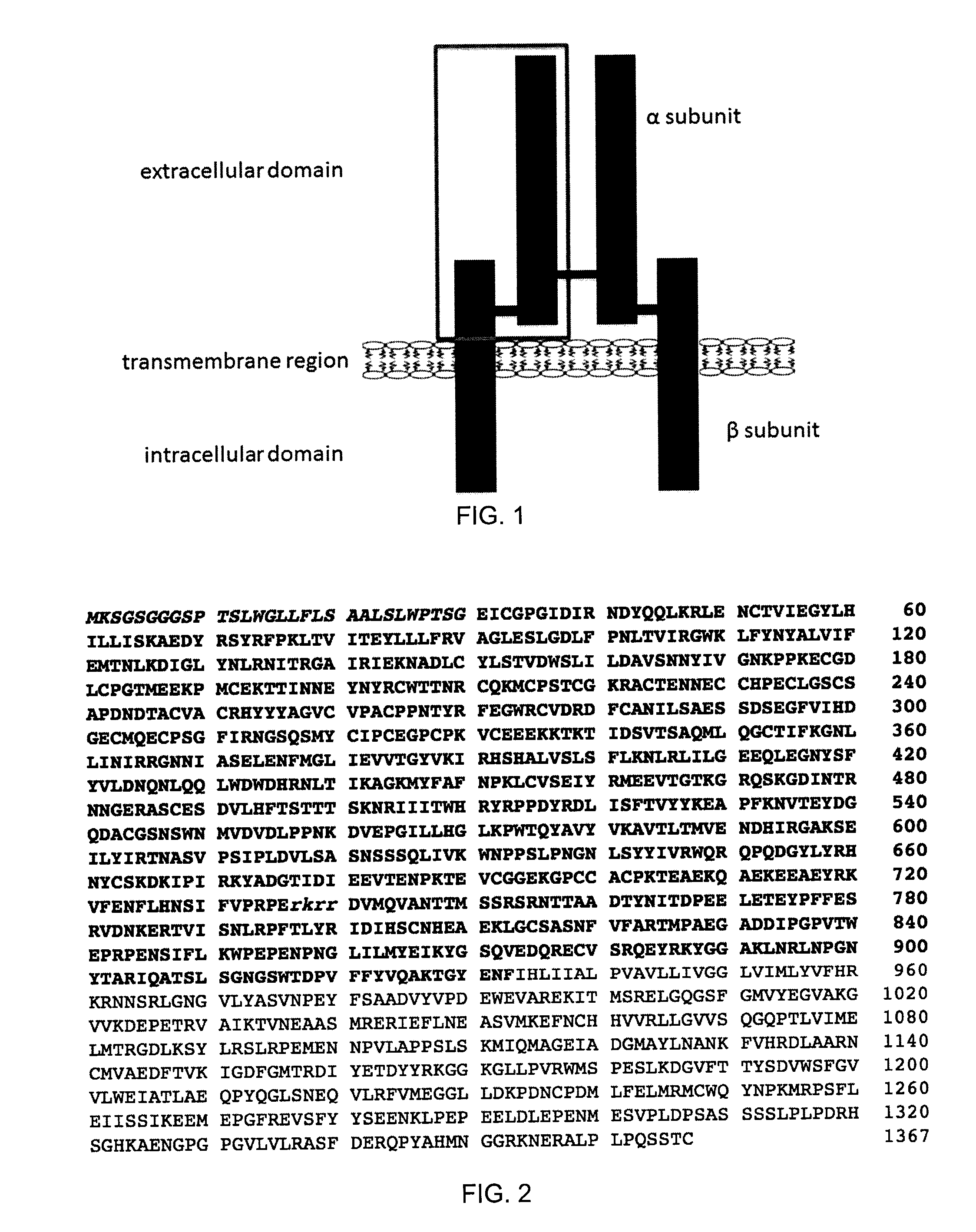
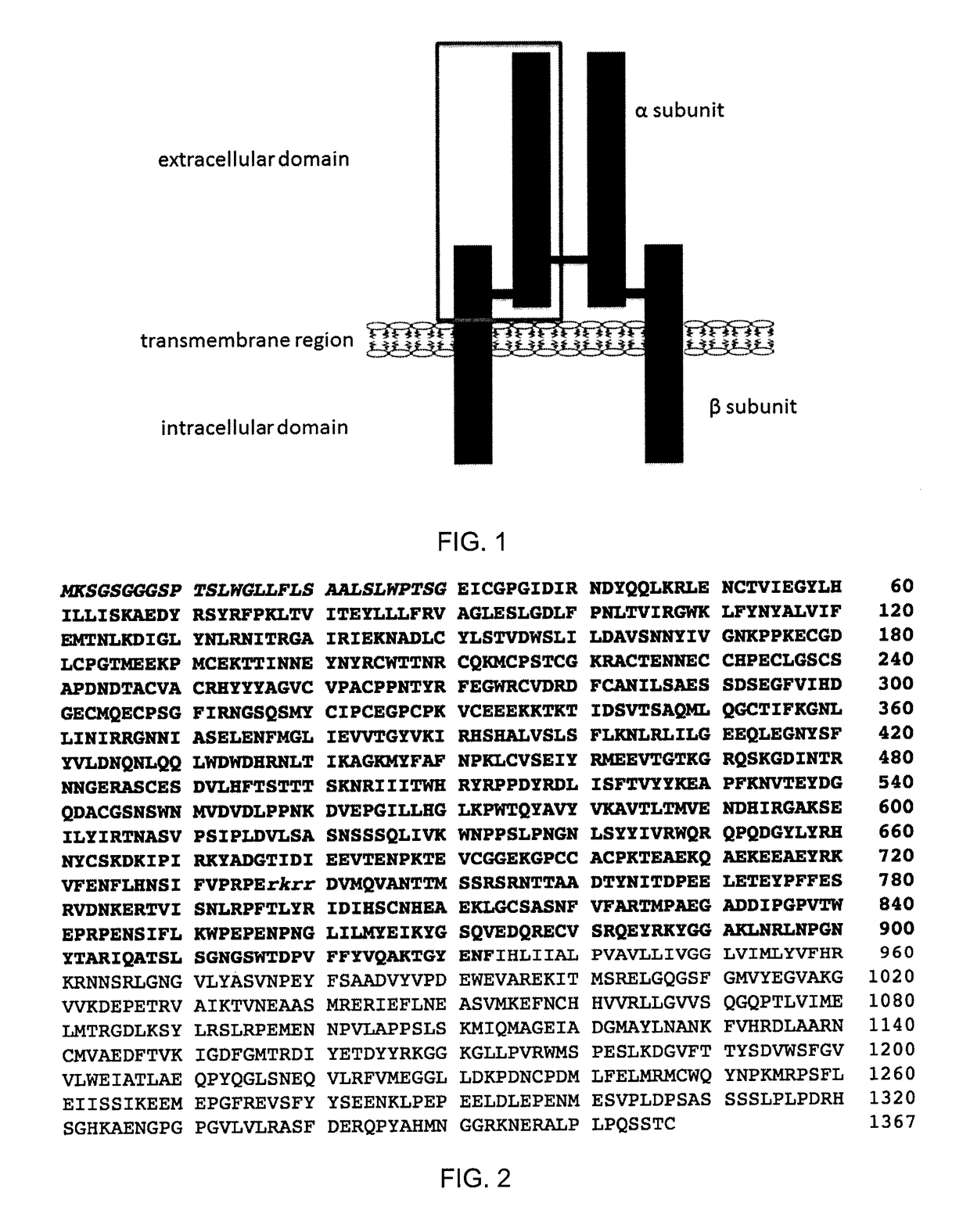
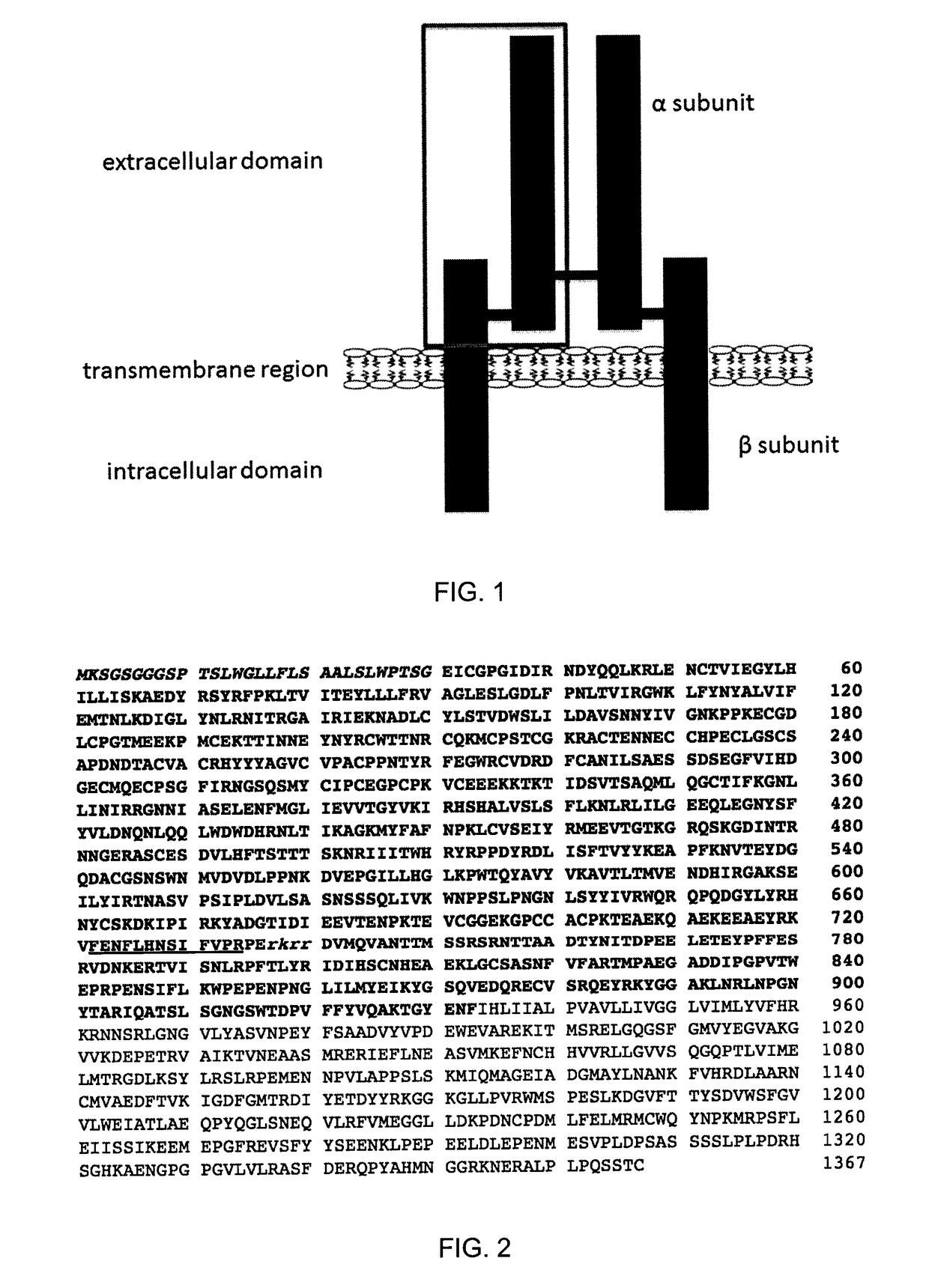
The insulin-like growth factor 1 (IGF-1) receptor is a protein found on the surface of human cells. It is a transmembrane receptor that is activated by a hormone called insulin-like growth factor 1 (IGF-1) and by a related hormone called IGF-2. It belongs to the large class of tyrosine kinase receptors. This receptor mediates the effects of IGF-1, which is a polypeptide protein hormone similar in molecular structure to insulin. IGF-1 plays an important role in growth and continues to have anabolic effects in adults – meaning that it can induce hypertrophy of skeletal muscle and other target tissues. Mice lacking the IGF-1 receptor die late in development, and show a dramatic reduction in body mass, testifying to the strong growth-promoting effect of this receptor.
Antibodies against insulin-like growth factor 1 receptor and uses thereof
ActiveUS20050008642A1Slow tumor growthExtension of timeImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsBiologyAntibody
Antibodies against insulin like growth factor I receptor (IGF-IR), methods for their production, pharmaceutical compositions containing said antibodies, and uses for such antibodies are disclosed. Such antibodies are implicated in antitumor therapy.
Owner:F HOFFMANN LA ROCHE & CO AG
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
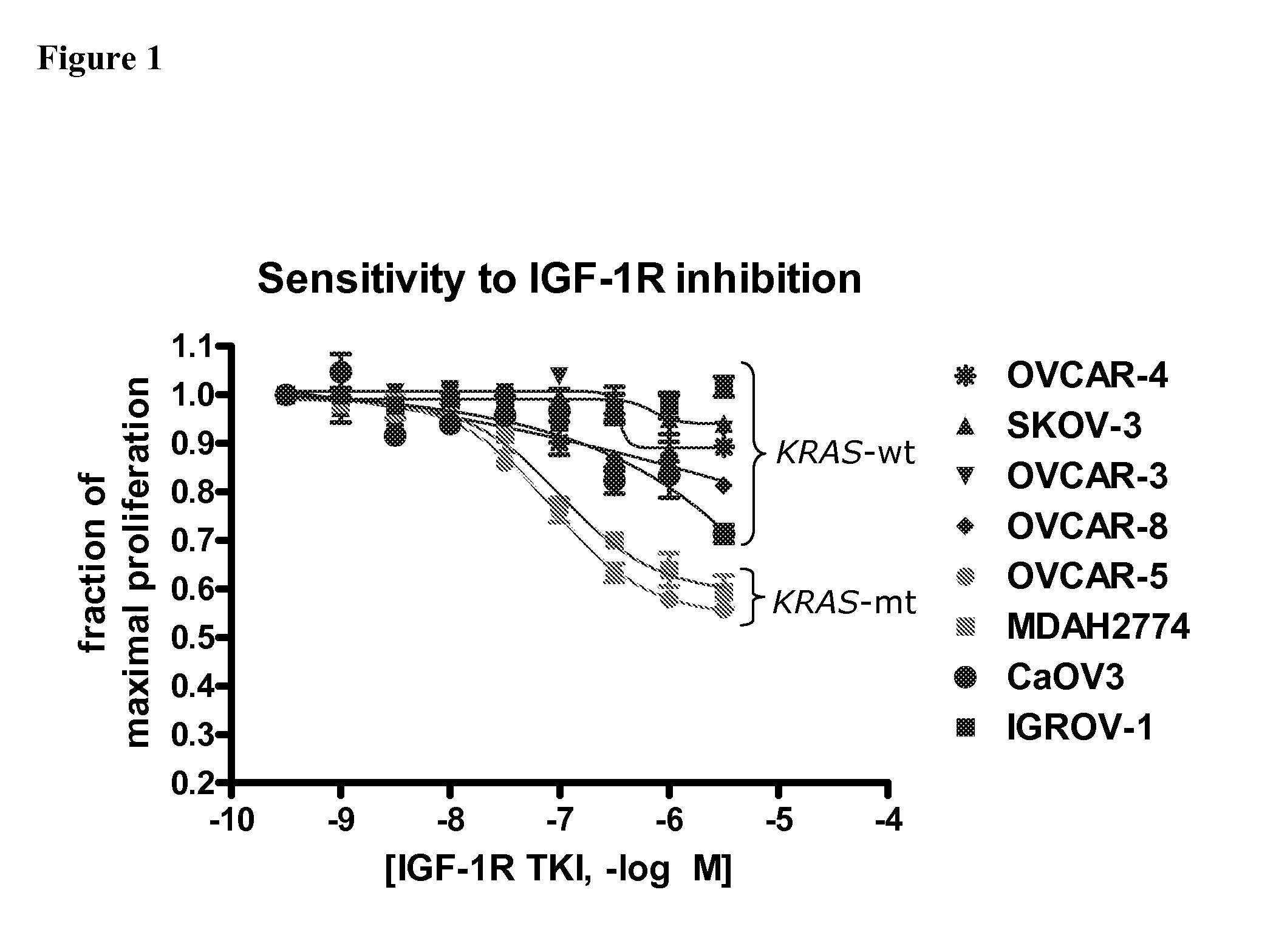
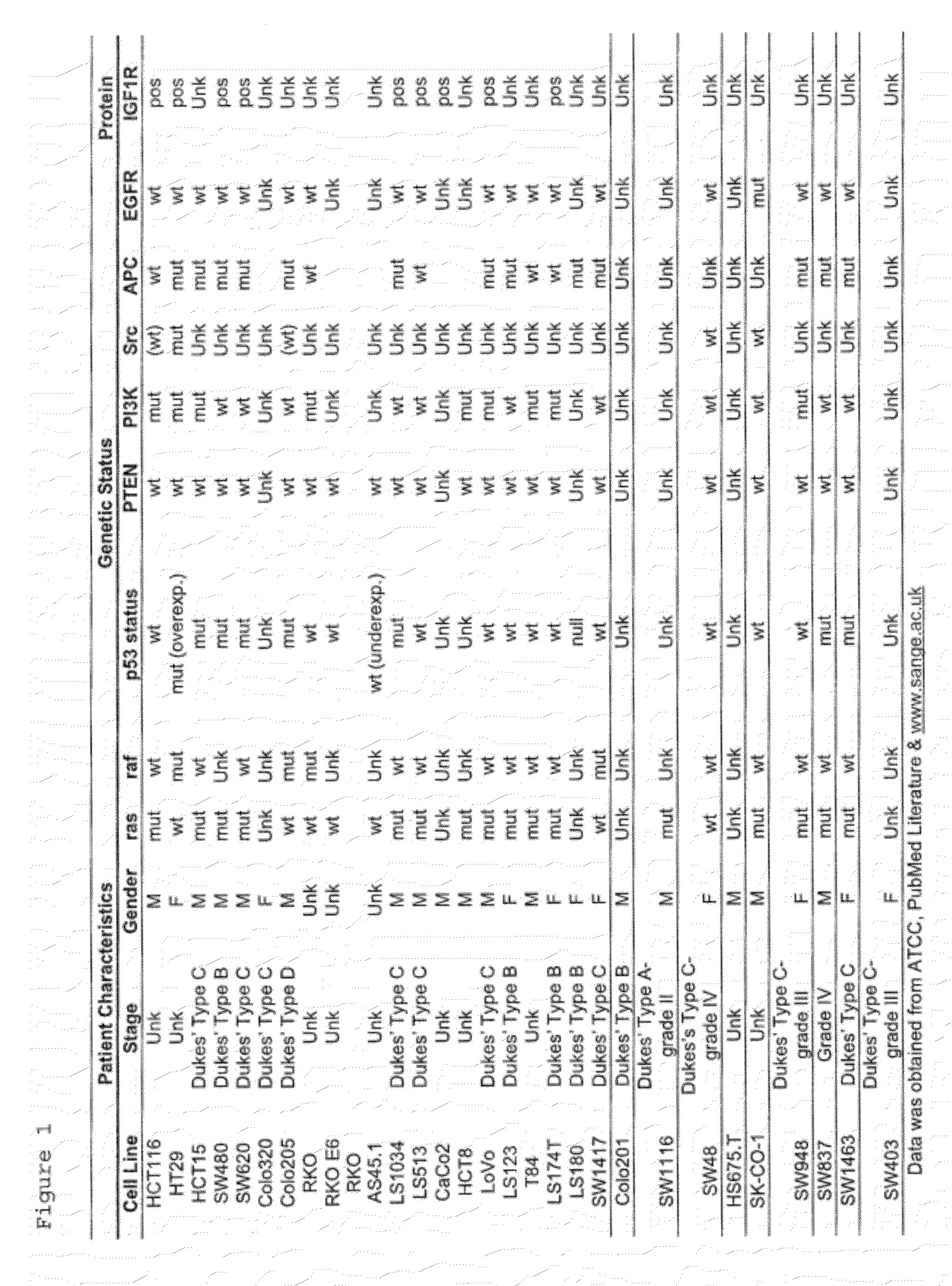
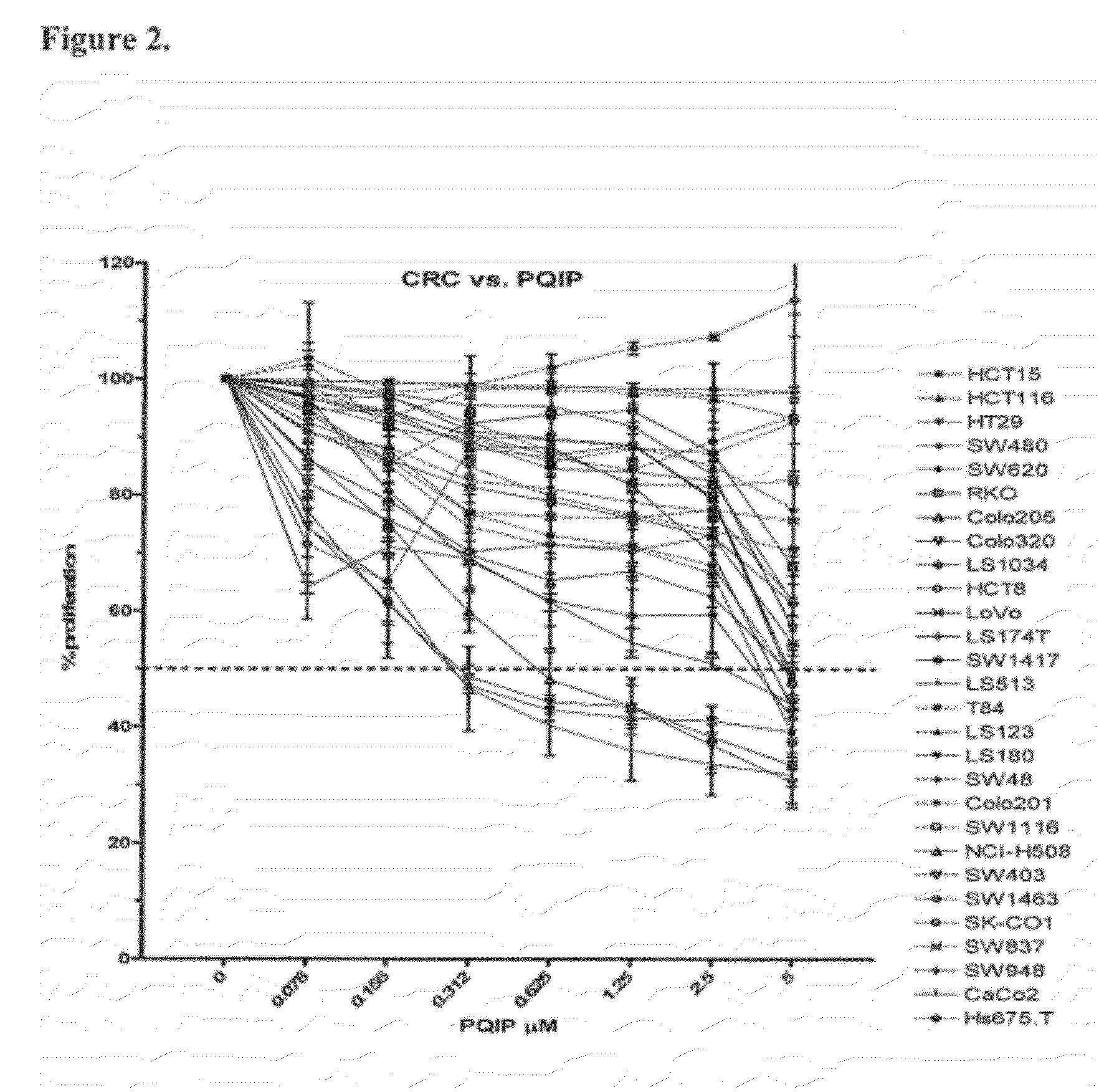
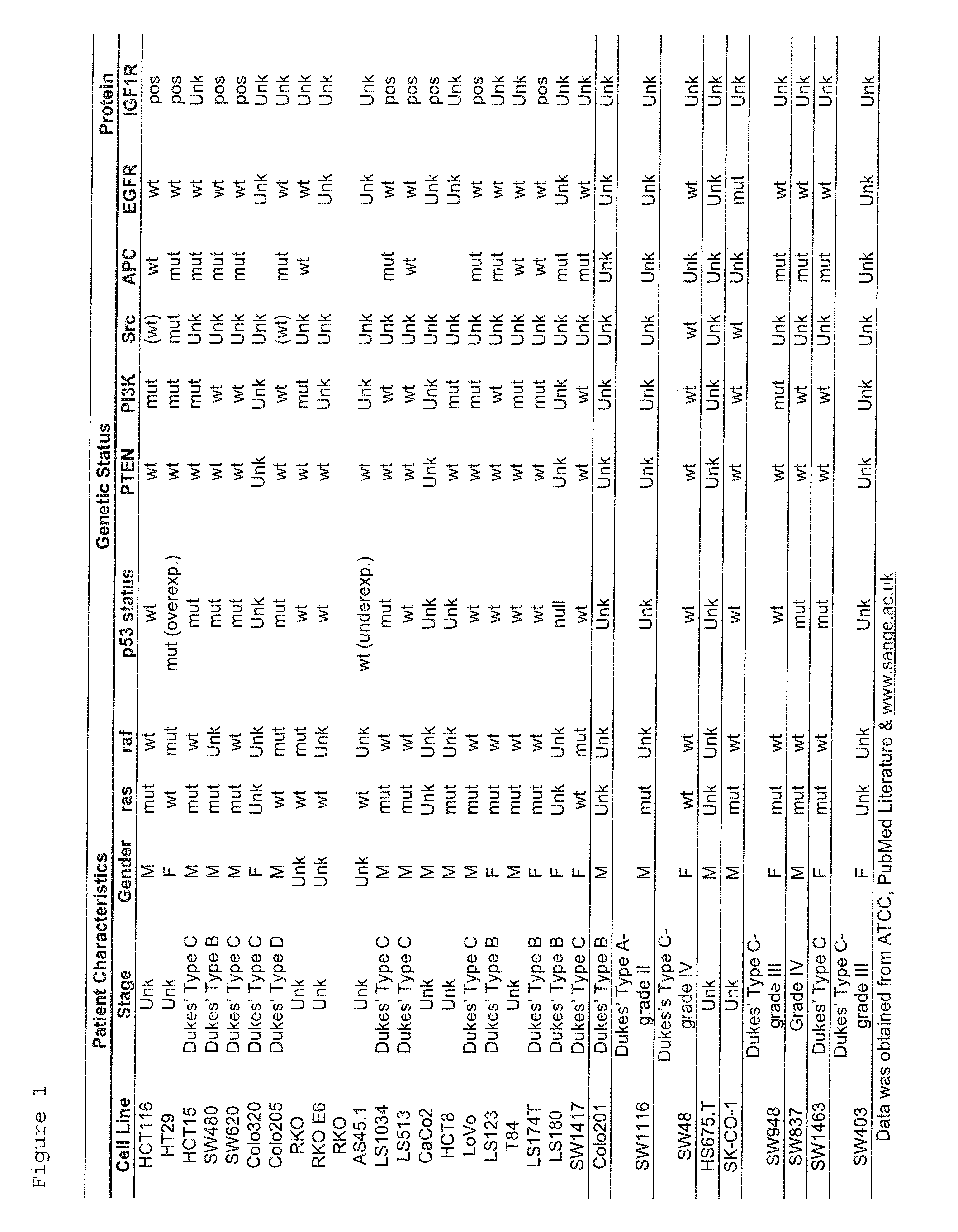
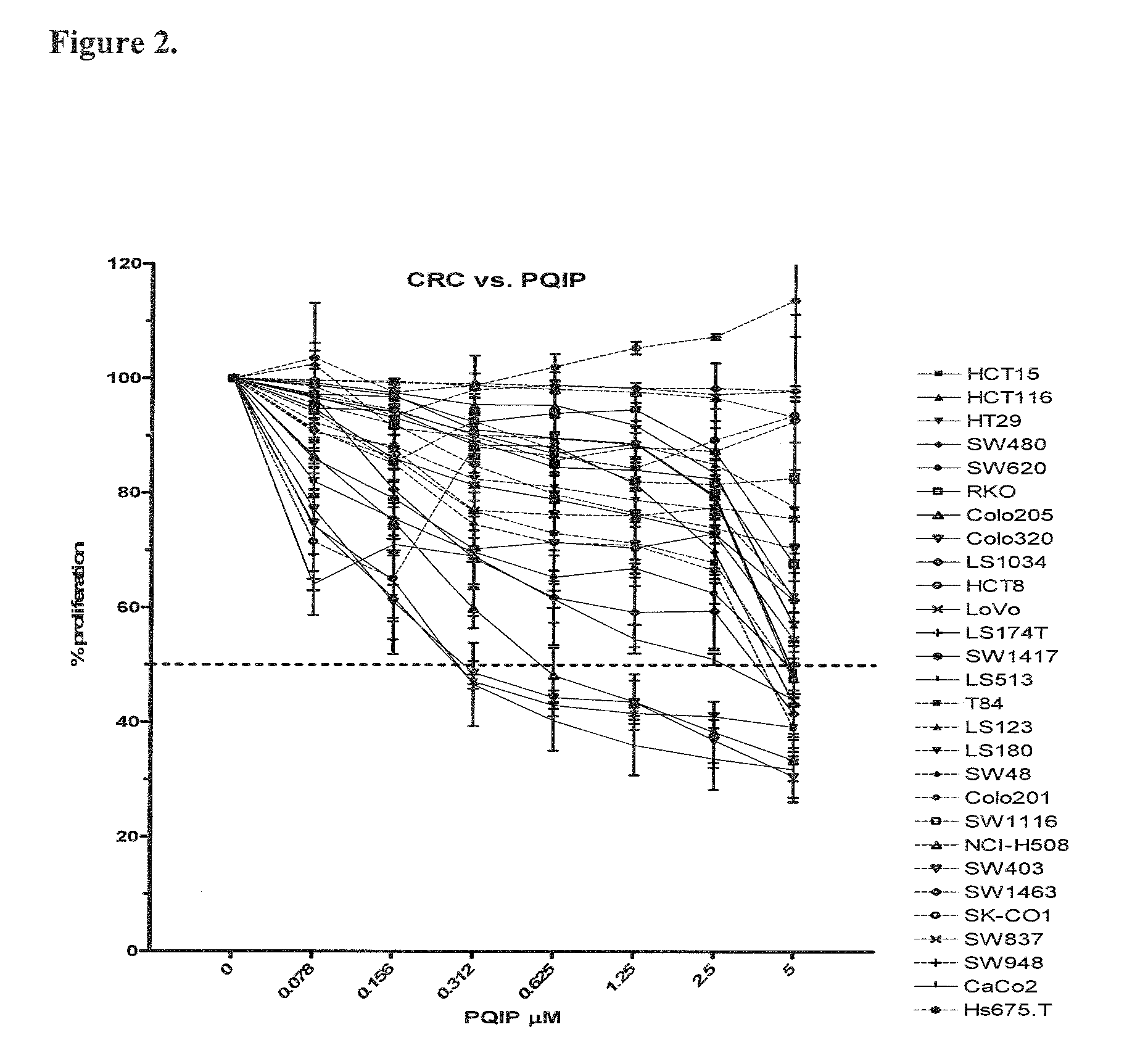
The present invention provides diagnostic methods for predicting the effectiveness of treatment of an ovarian cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cells possess mutant K-RAS. The present invention thus provides a method of identifying patients with ovarian cancer who are most likely to benefit from treatment with an IGF-1R kinase inhibitor. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate this methodology are also provided. The present invention also provides diagnostic methods for predicting the effectiveness of treatment of cancer patients with IGF-1R kinase inhibitors, based on a determination of the mutation status of the genes K-RAS, B-RAF, PTEN and PIK3CA, which can be used to identify tumor cell types that will be sensitive to IGF-1R kinase inhibitors, and also those that will be insensitive.
Owner:OSI PHARMA INC
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
InactiveUS20090092596A1High level of biomarkerReduce sensitivityMicrobiological testing/measurementAntibody ingredientsCell growthWilms' tumor
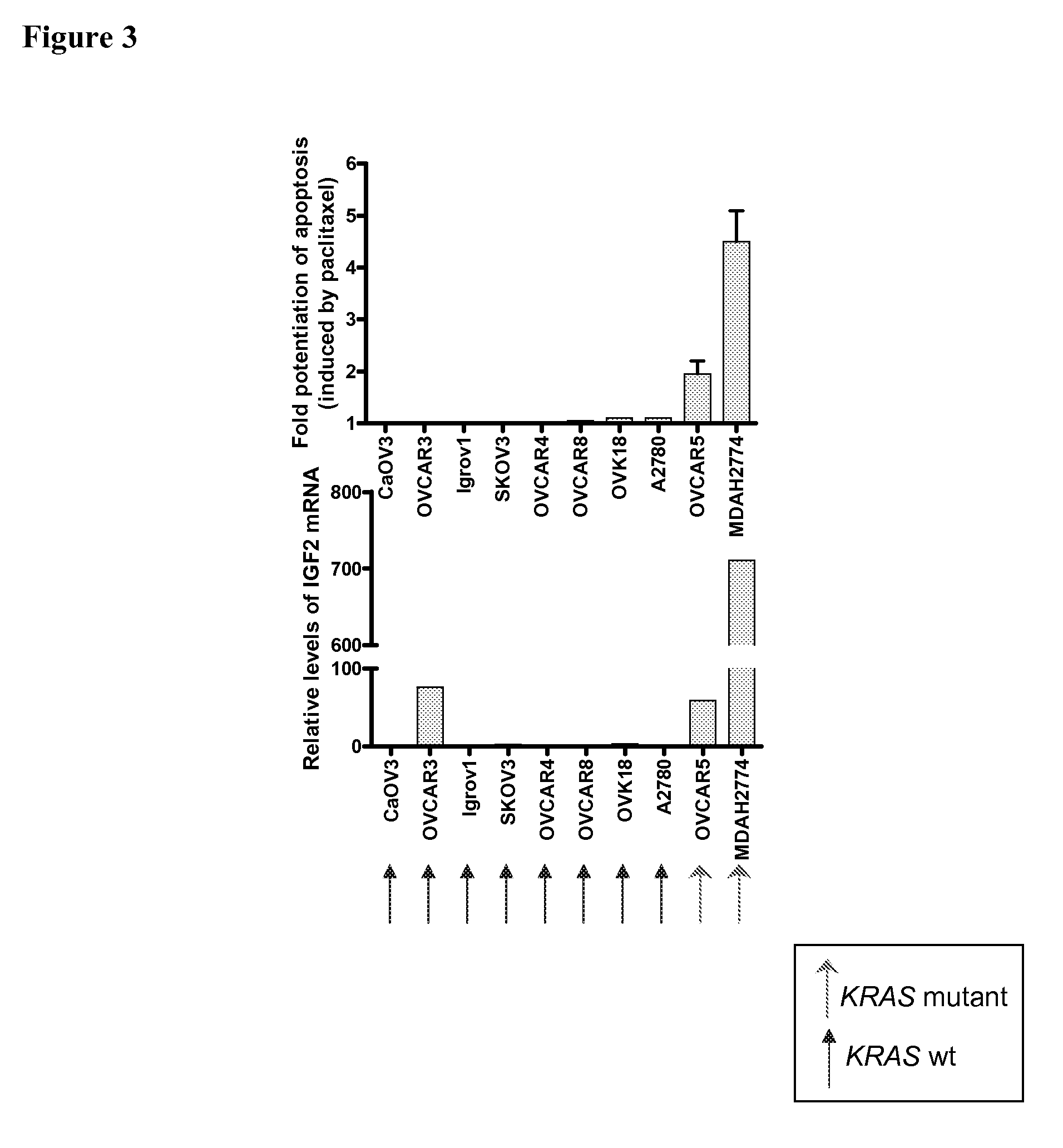
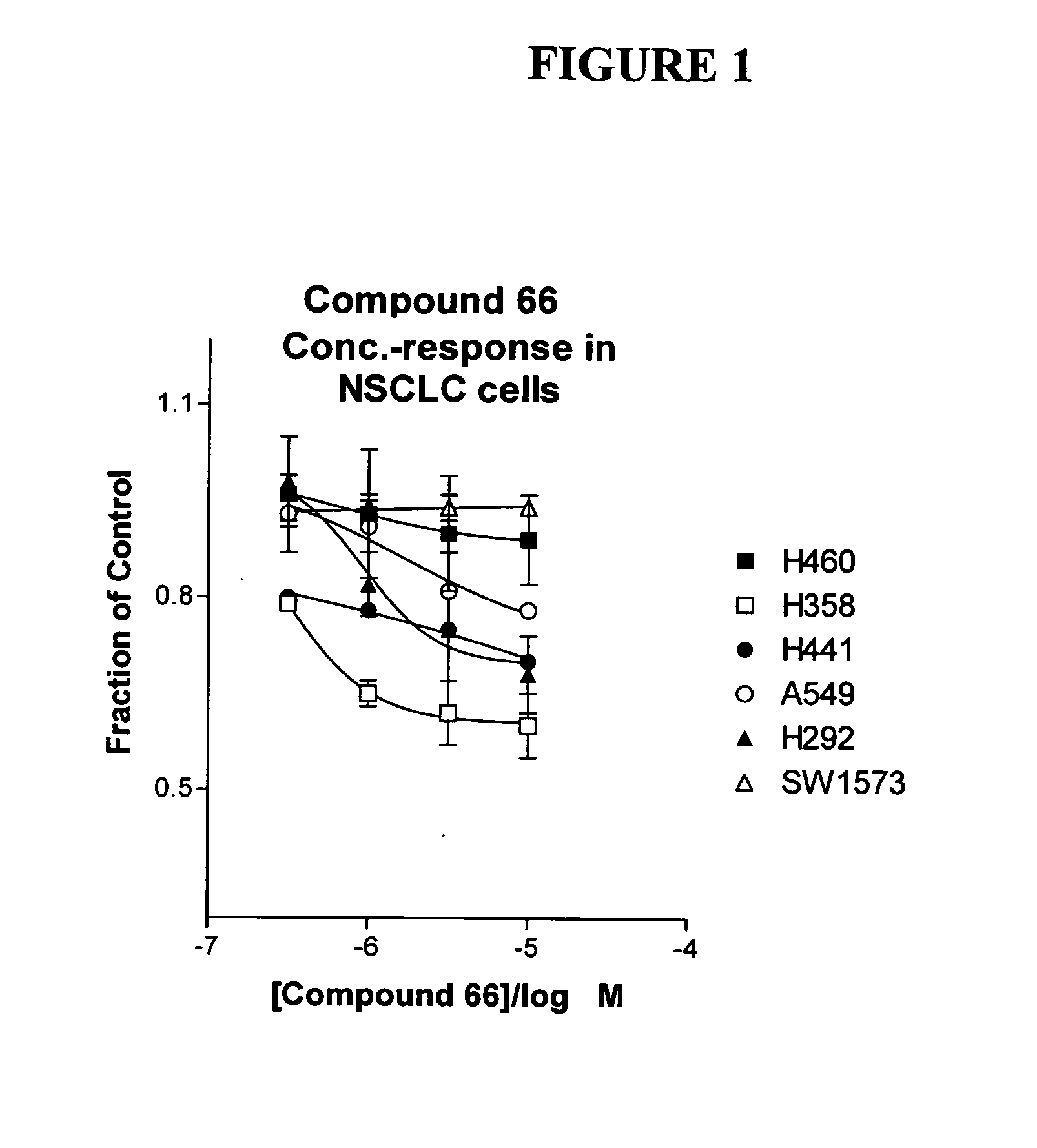
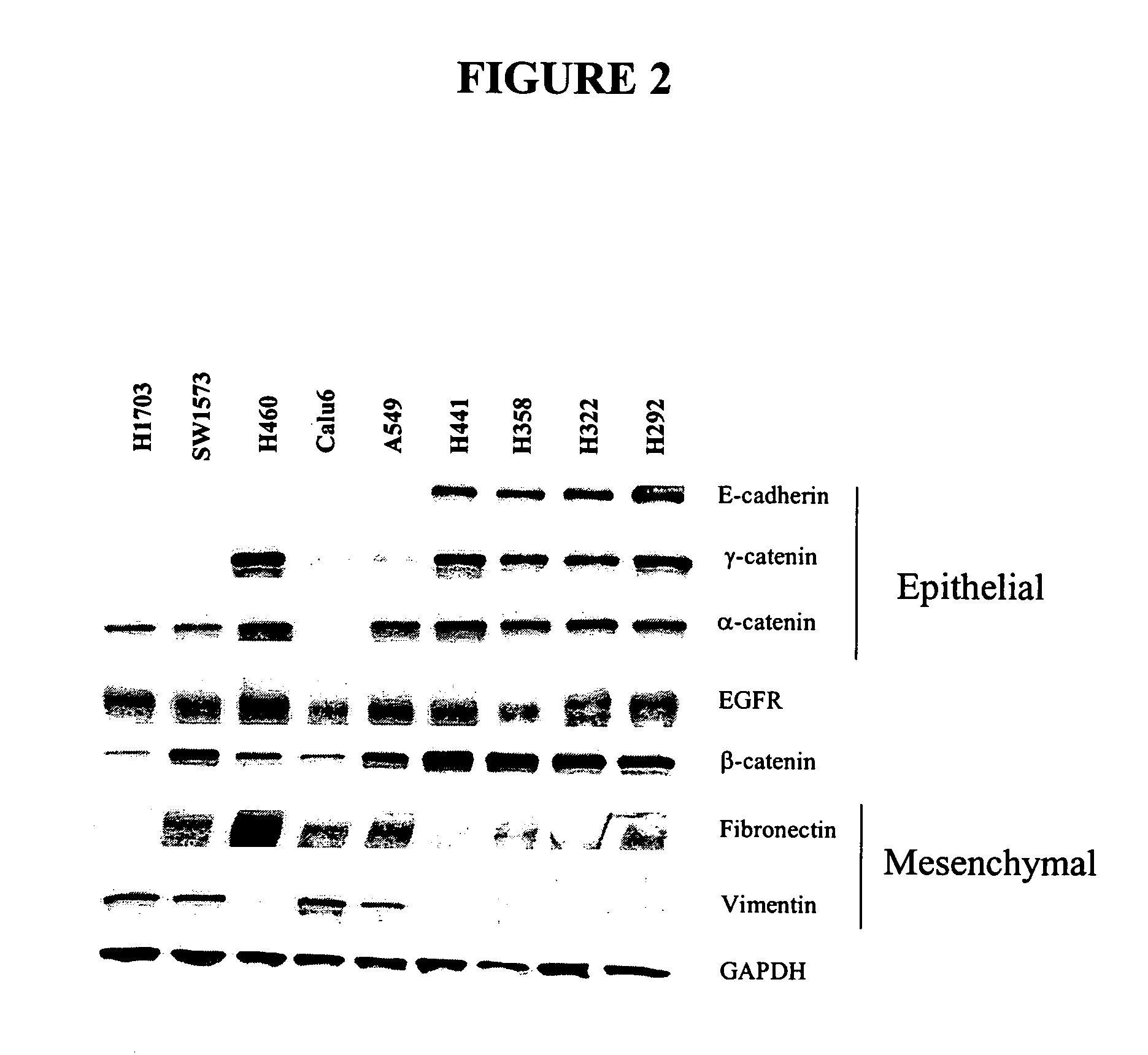
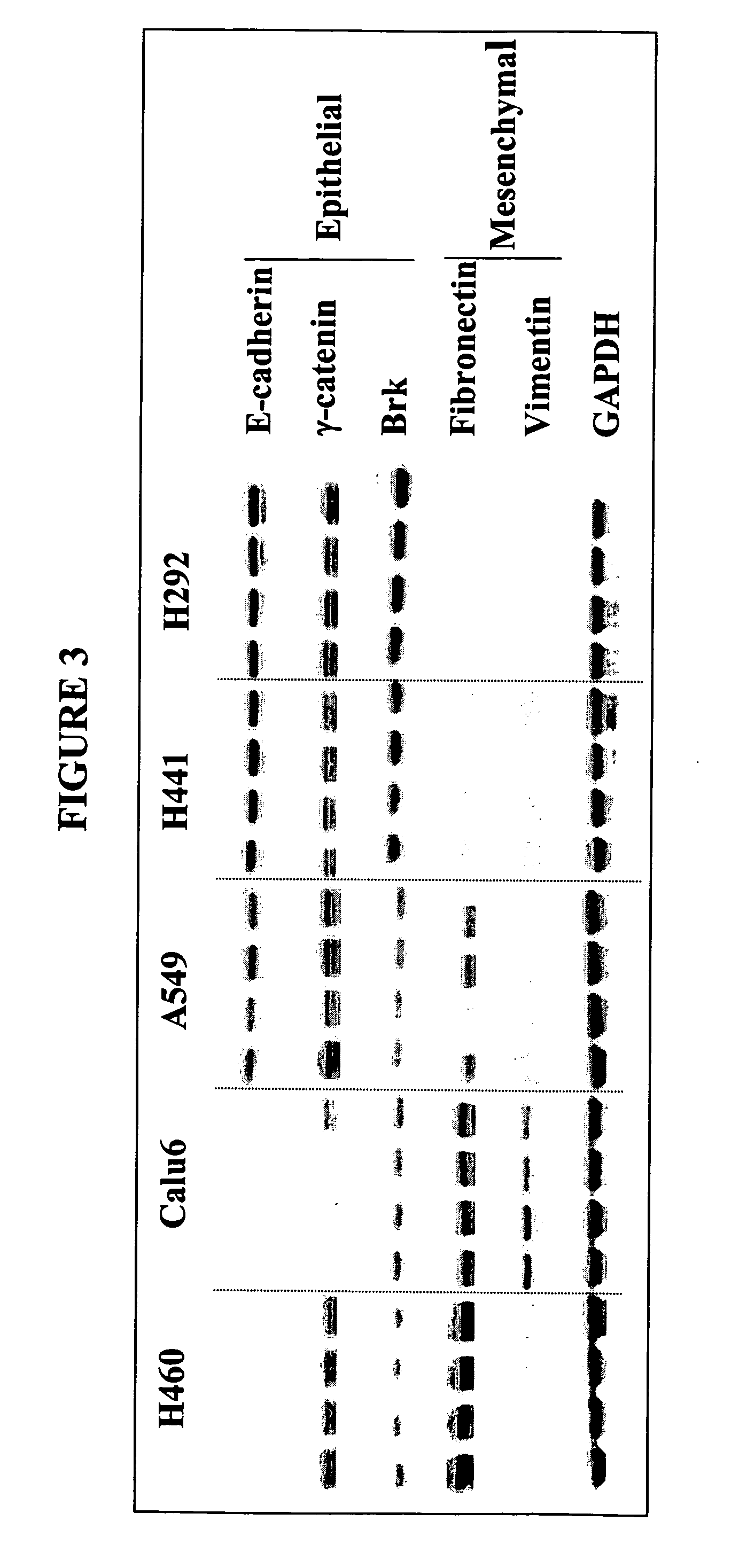
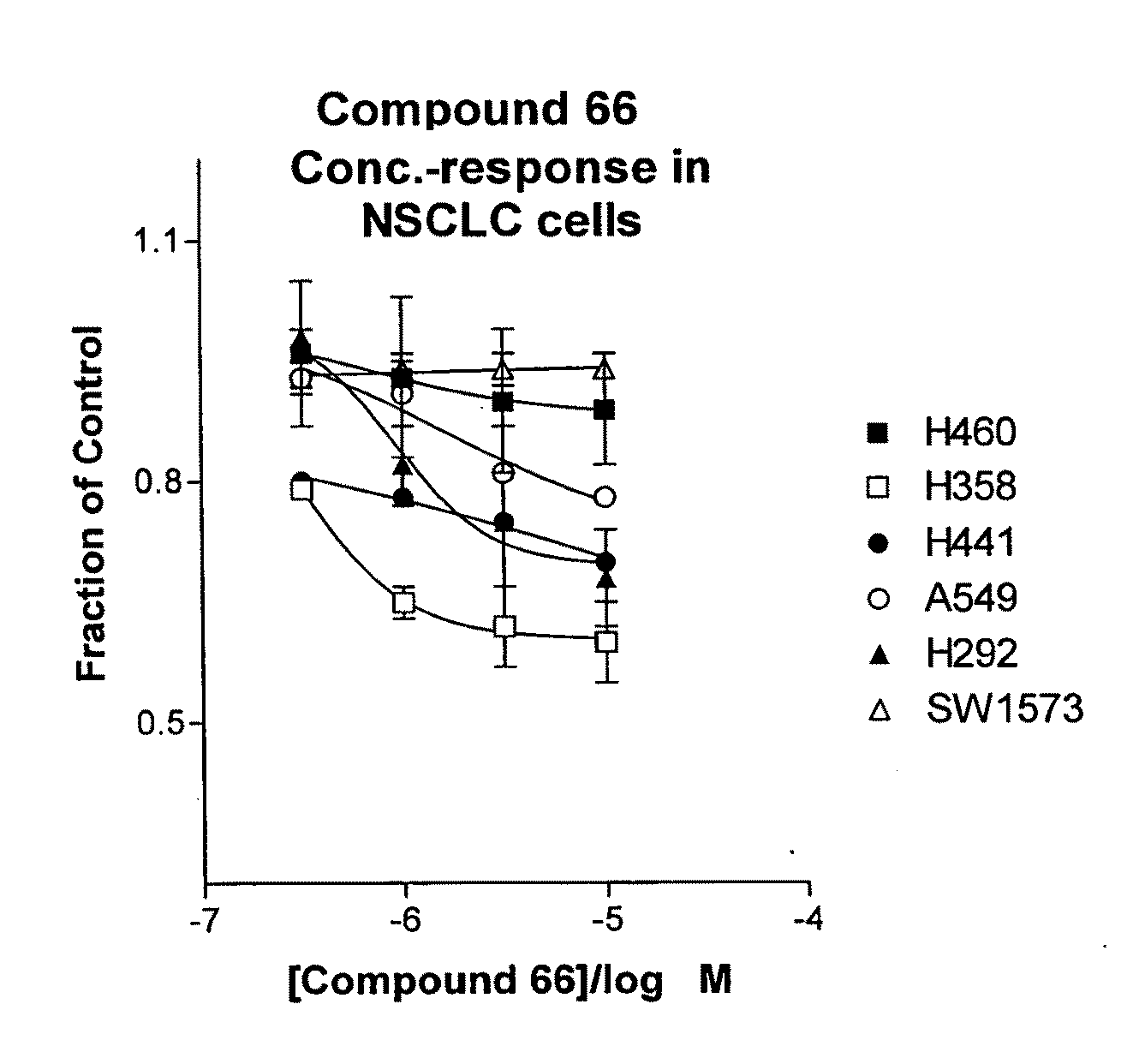
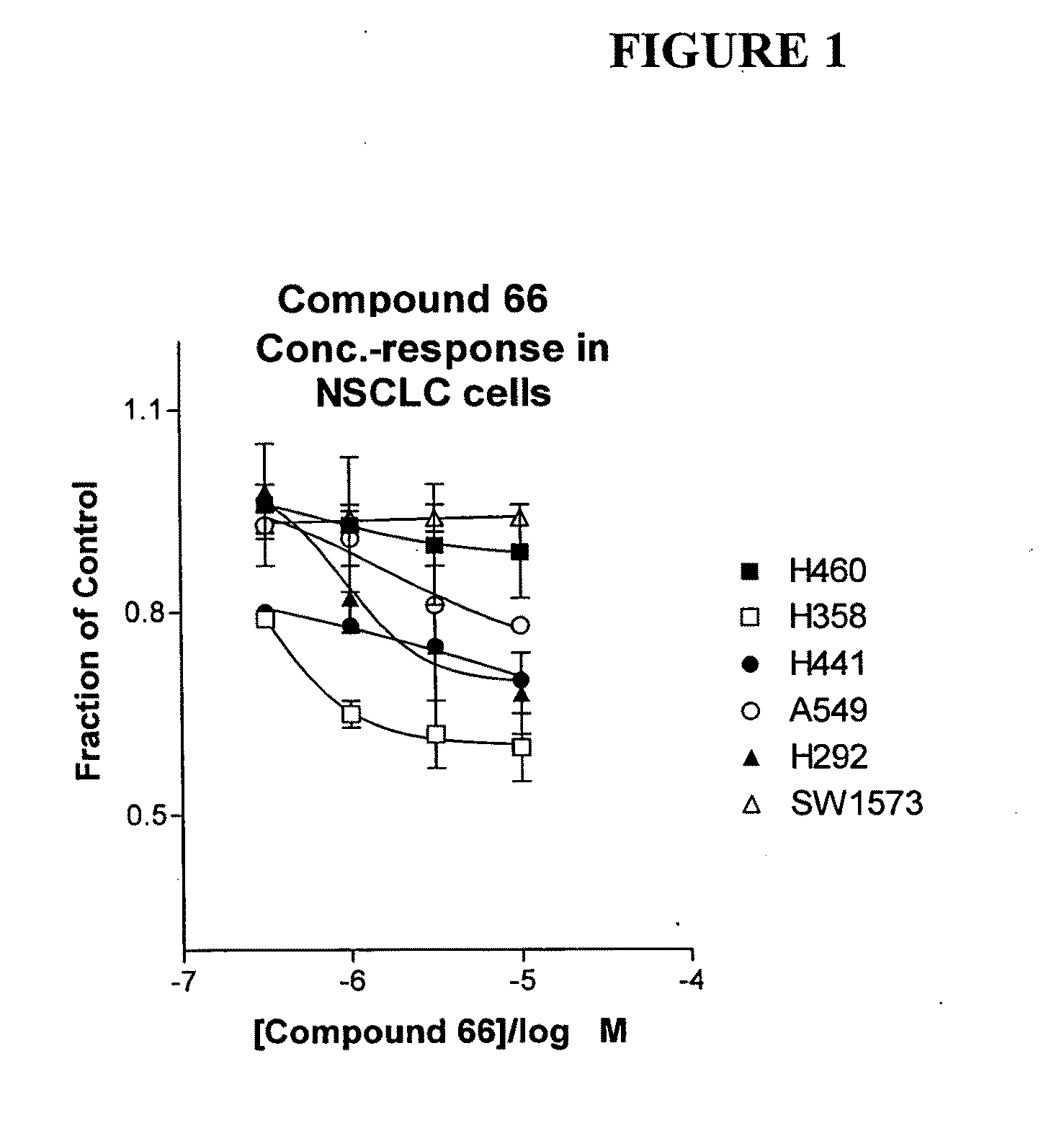
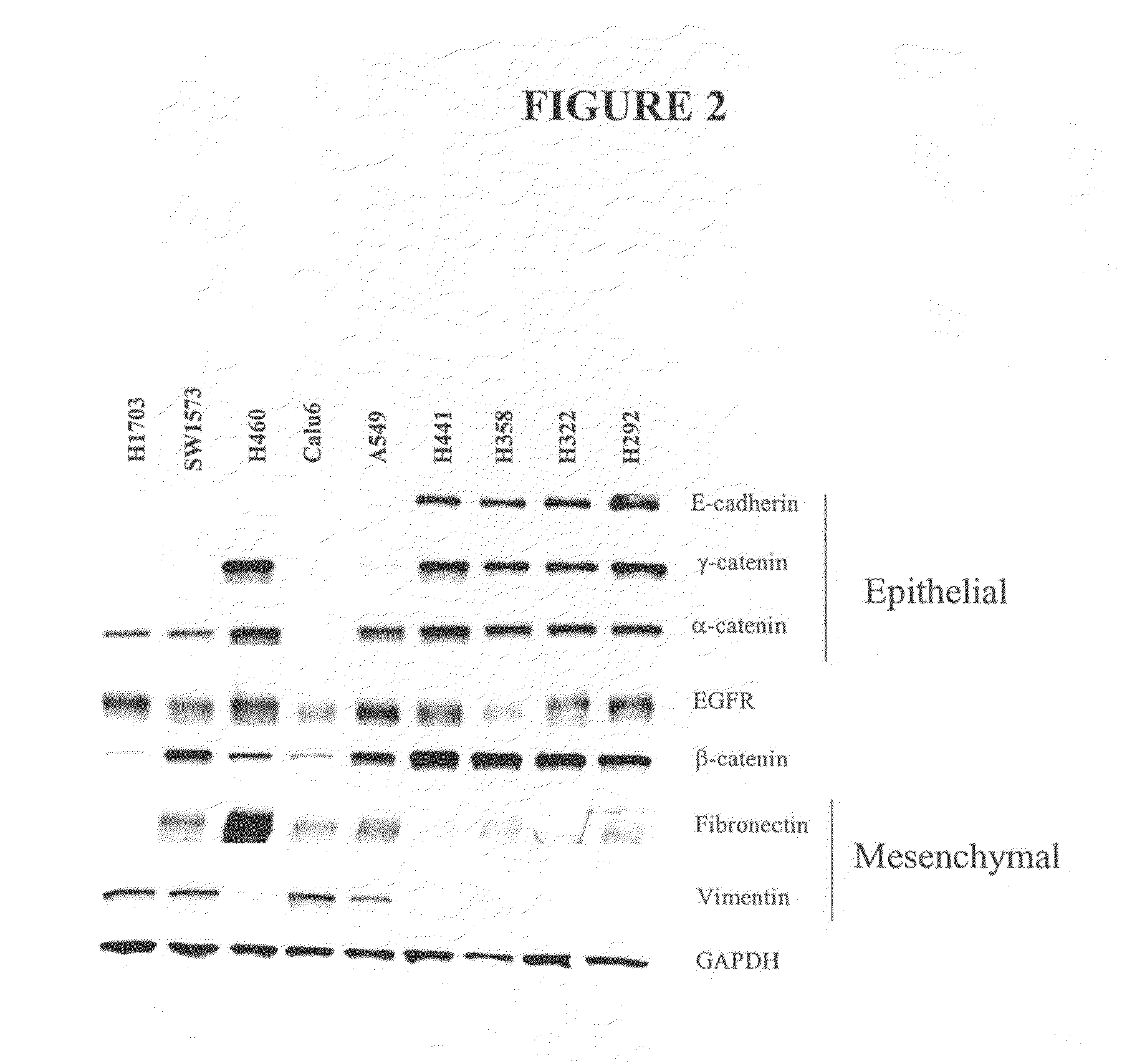
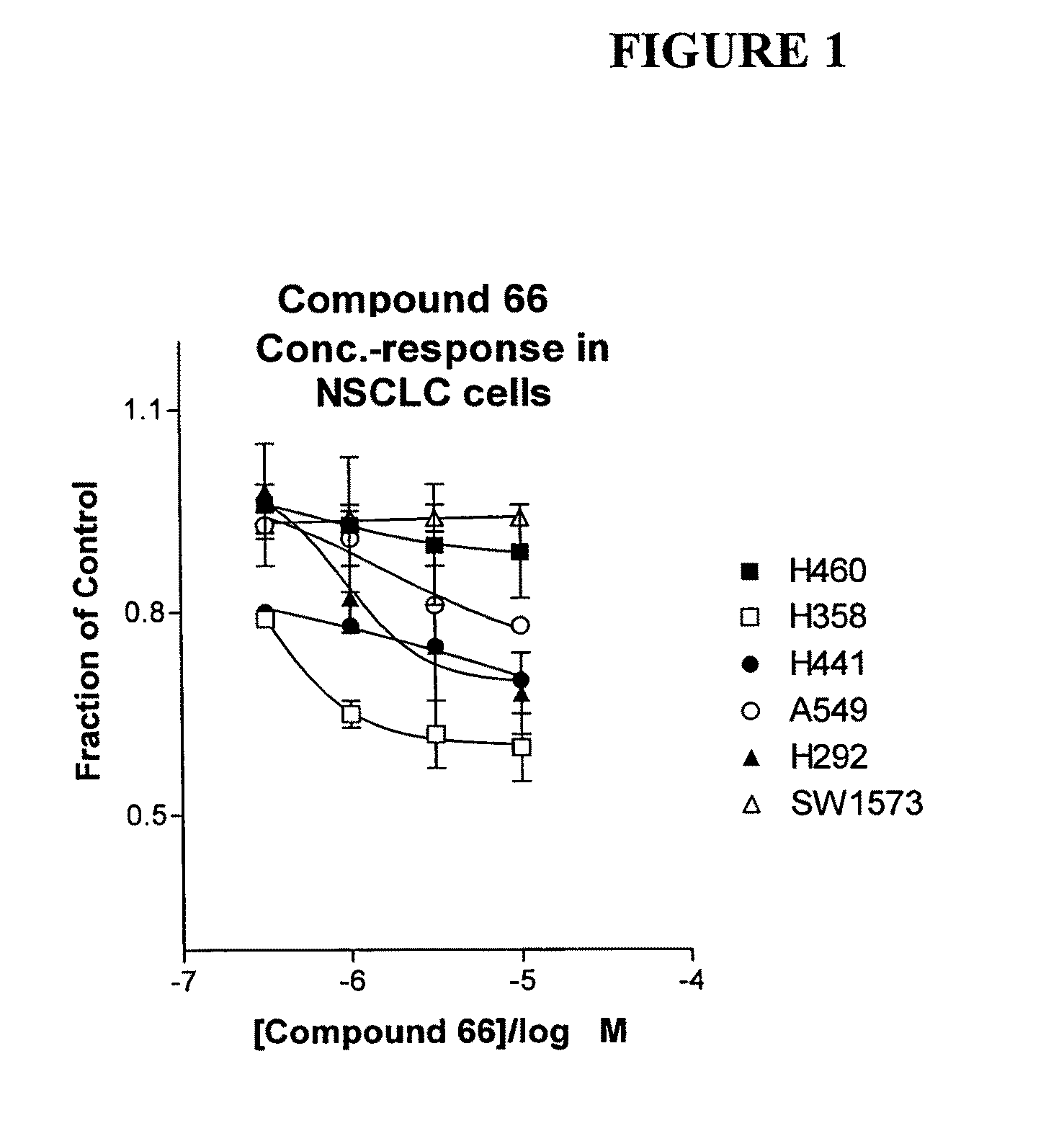
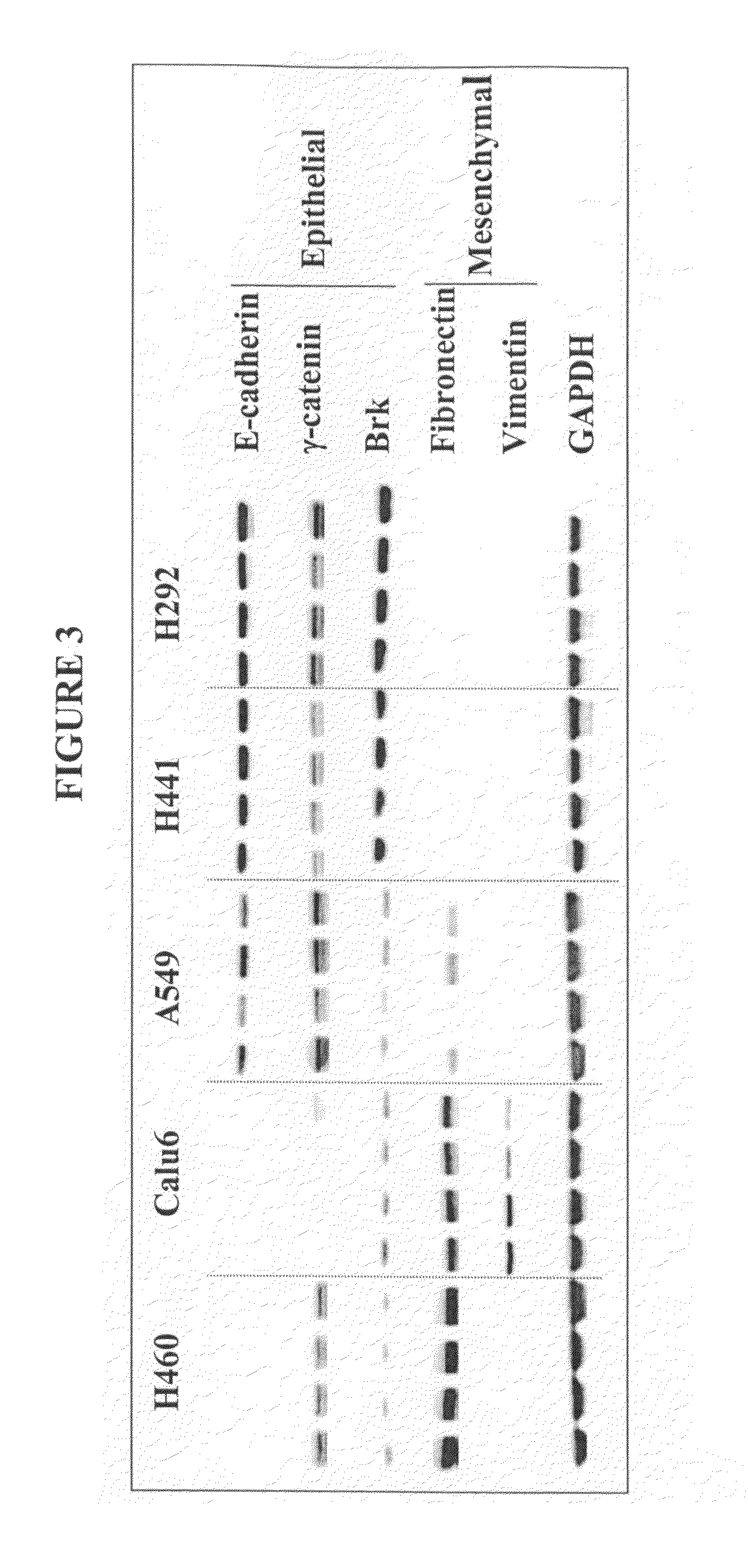
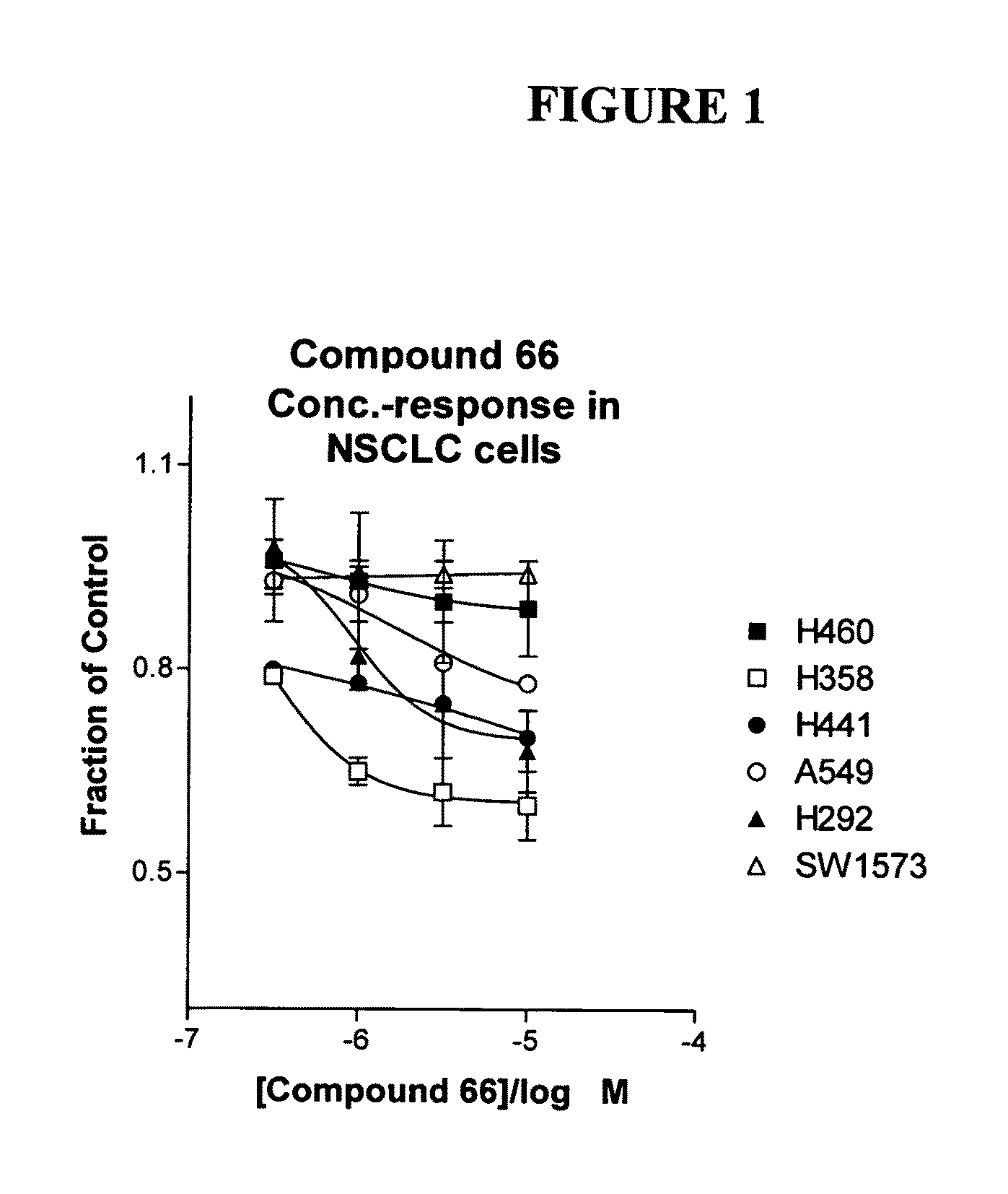
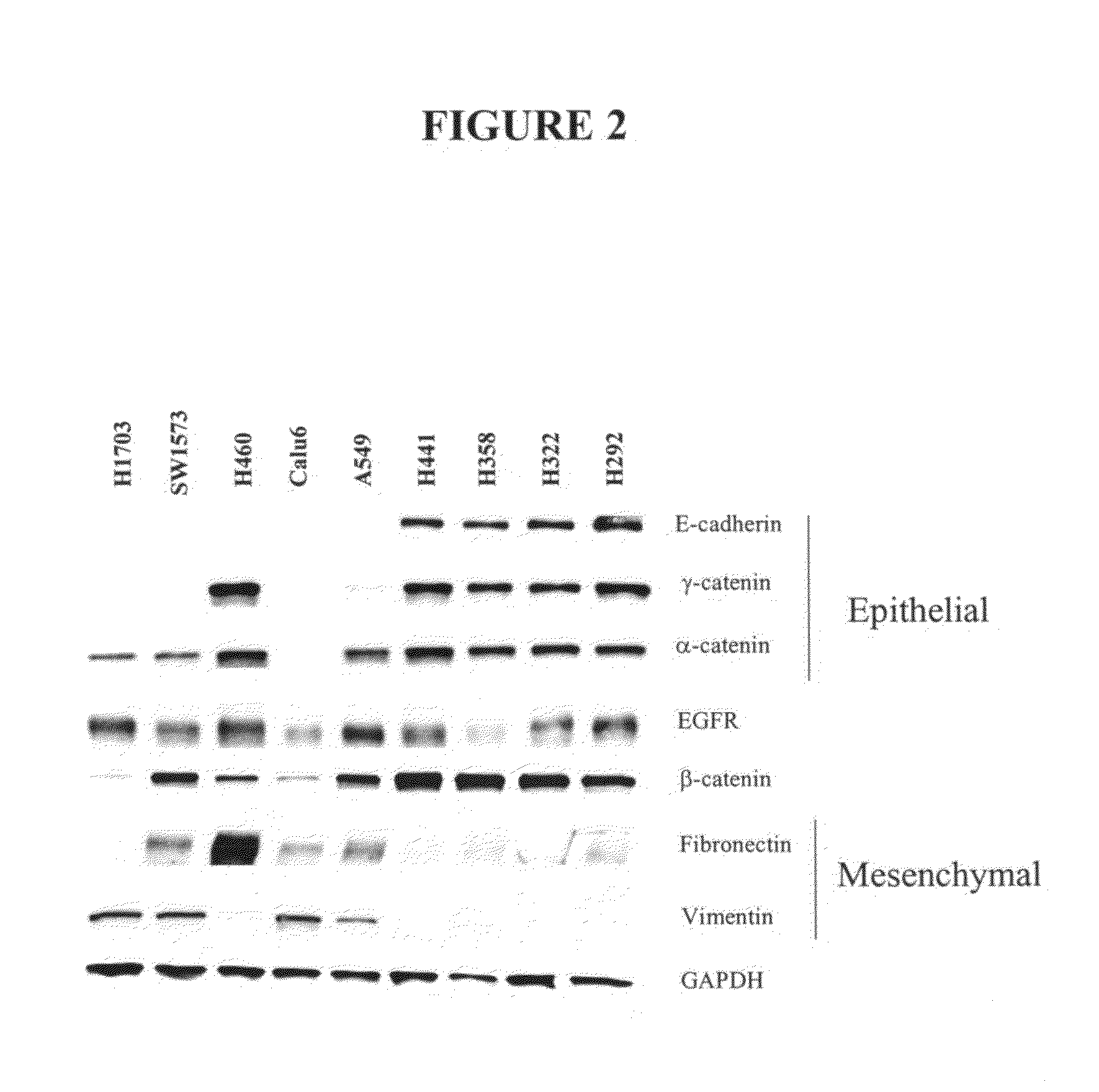
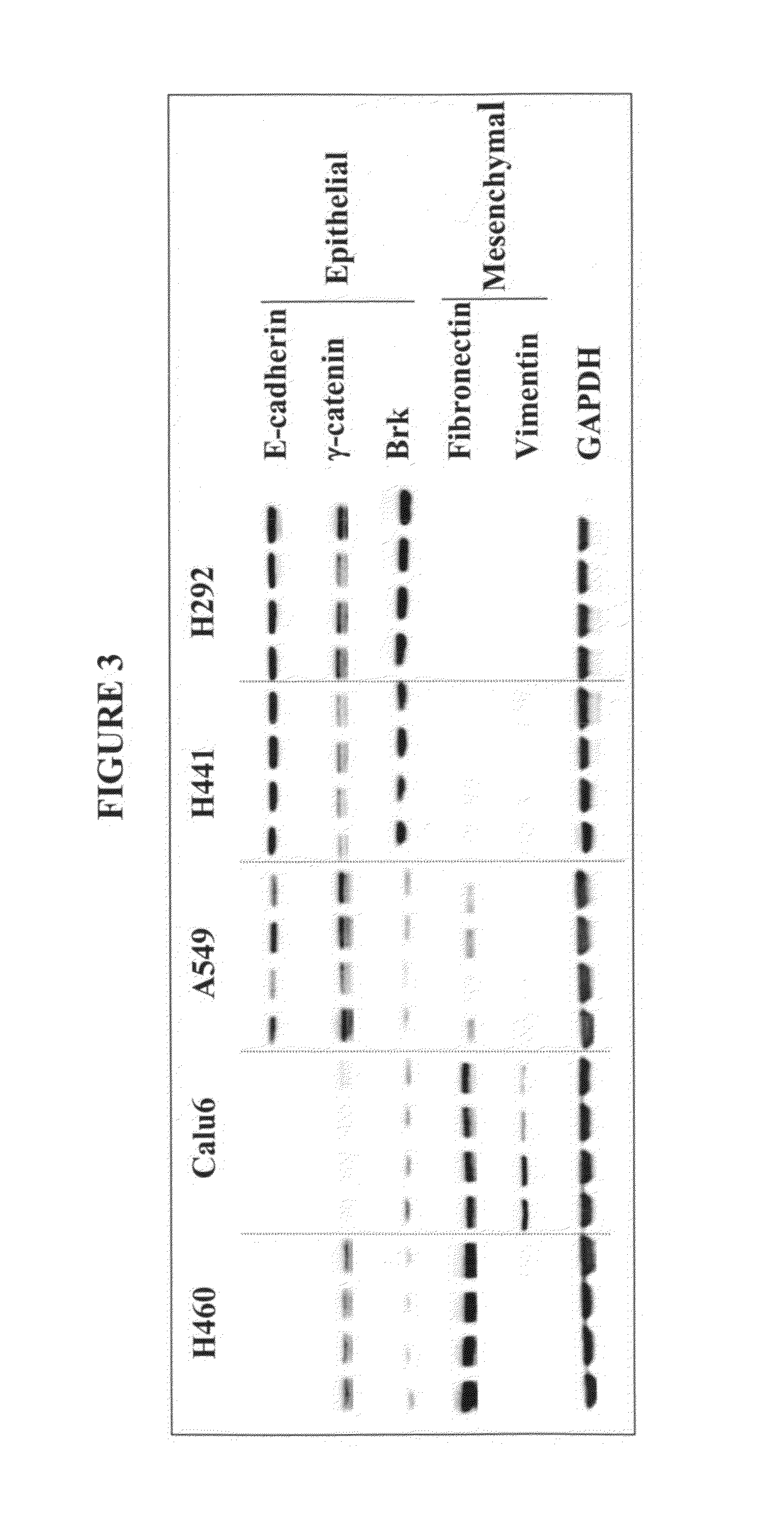
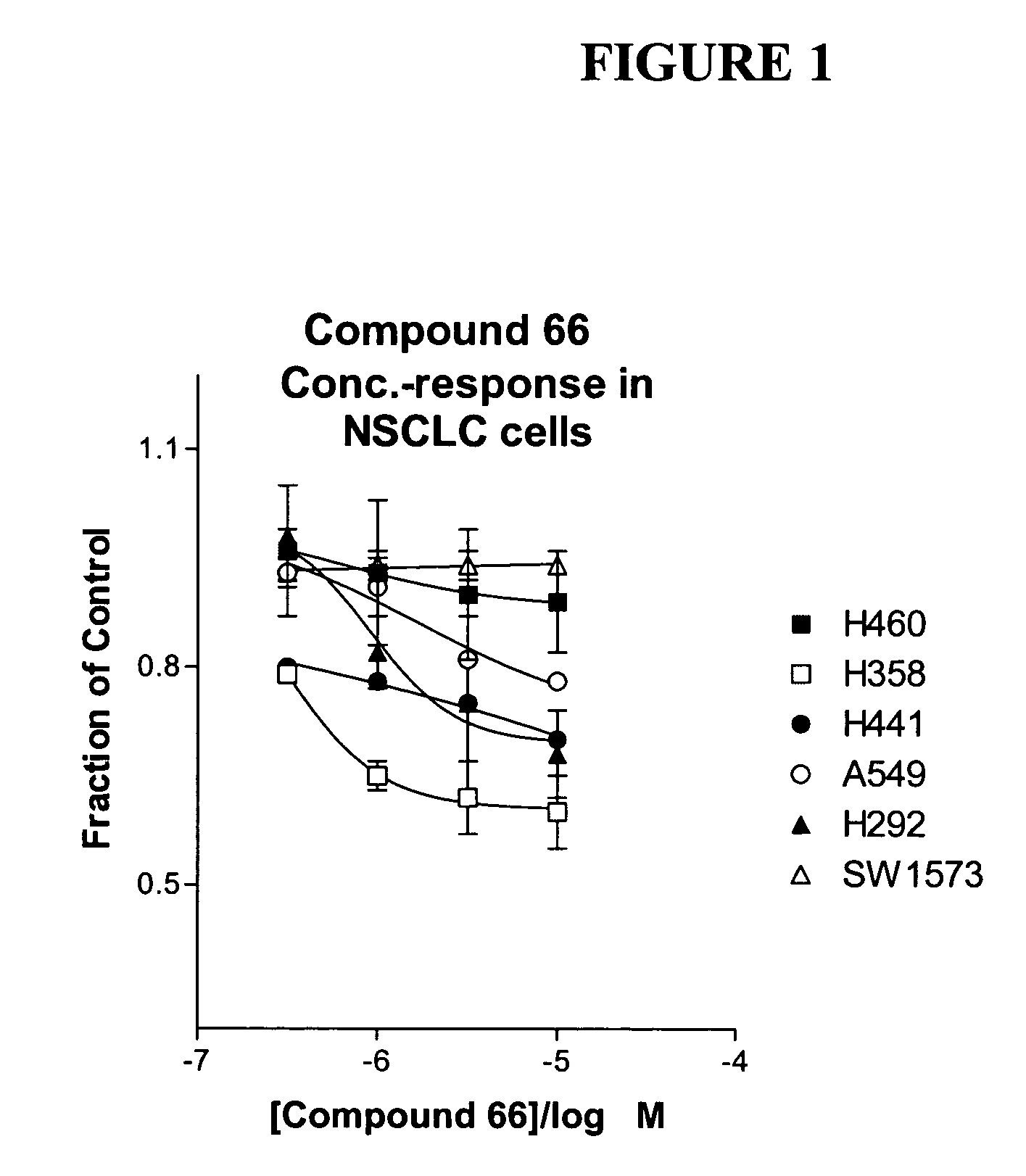
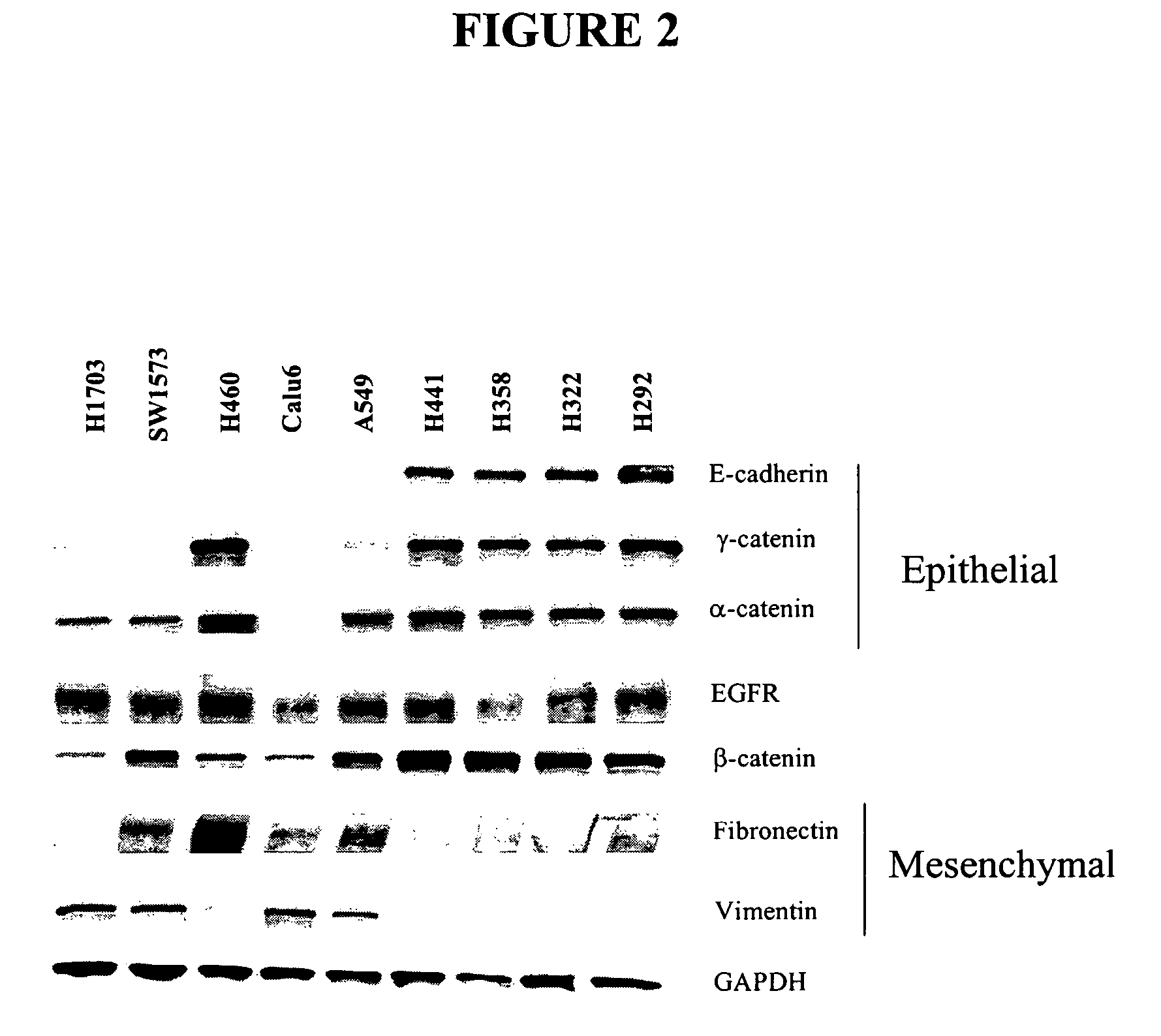
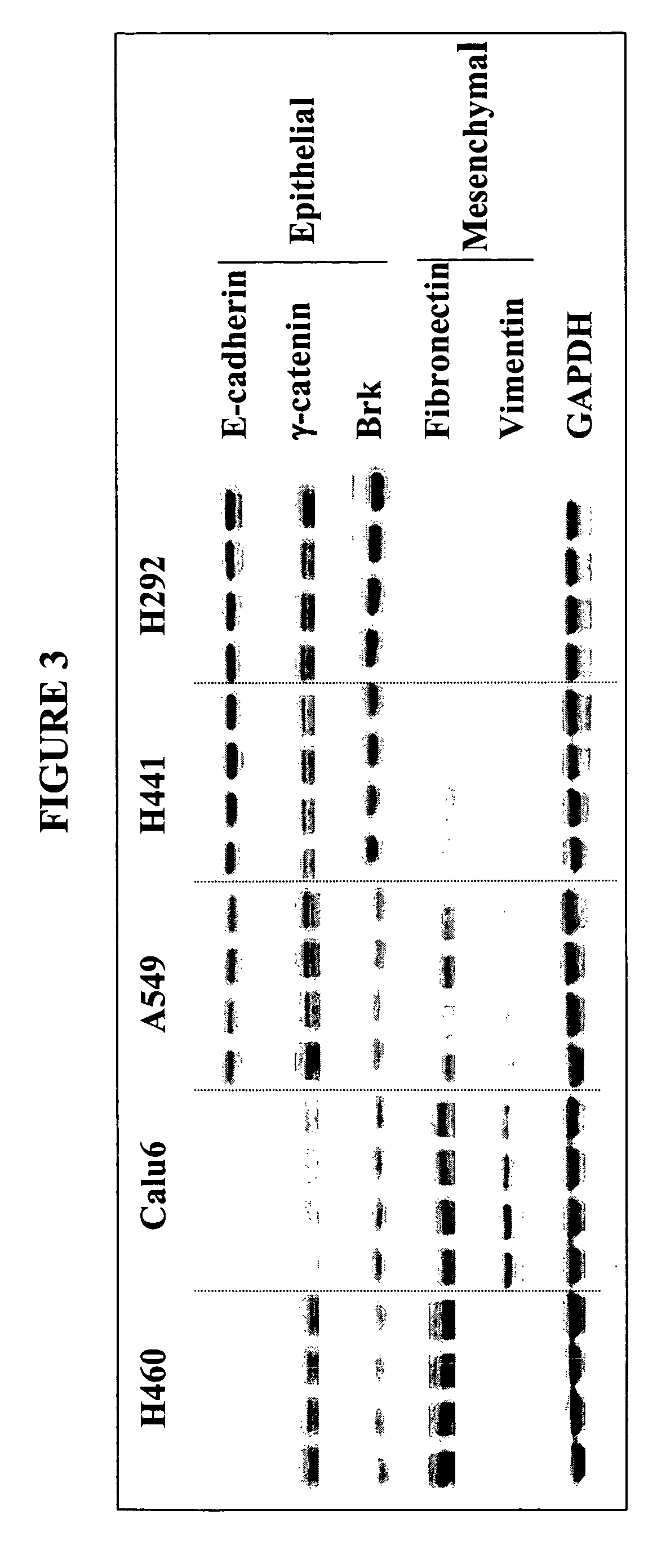
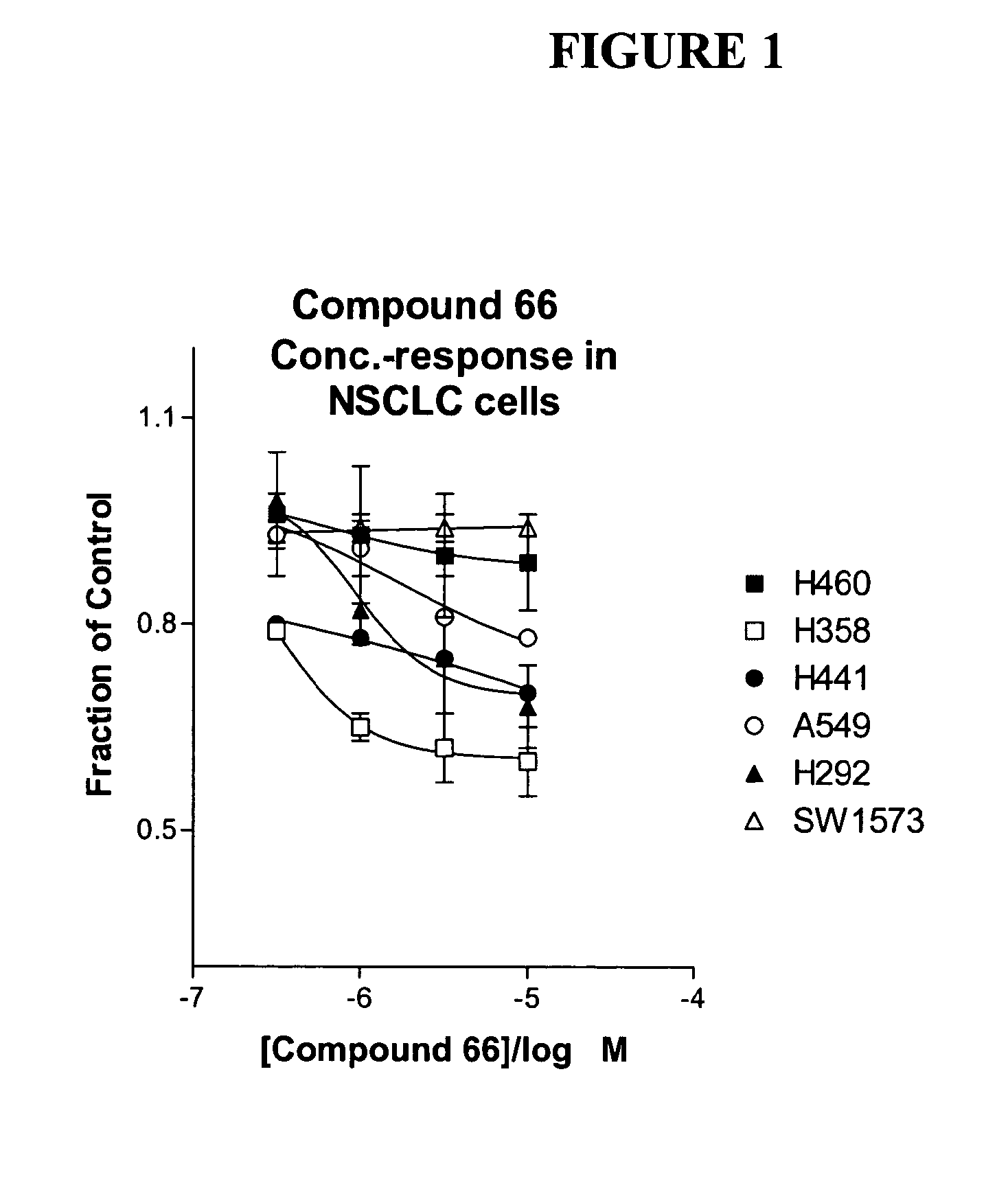
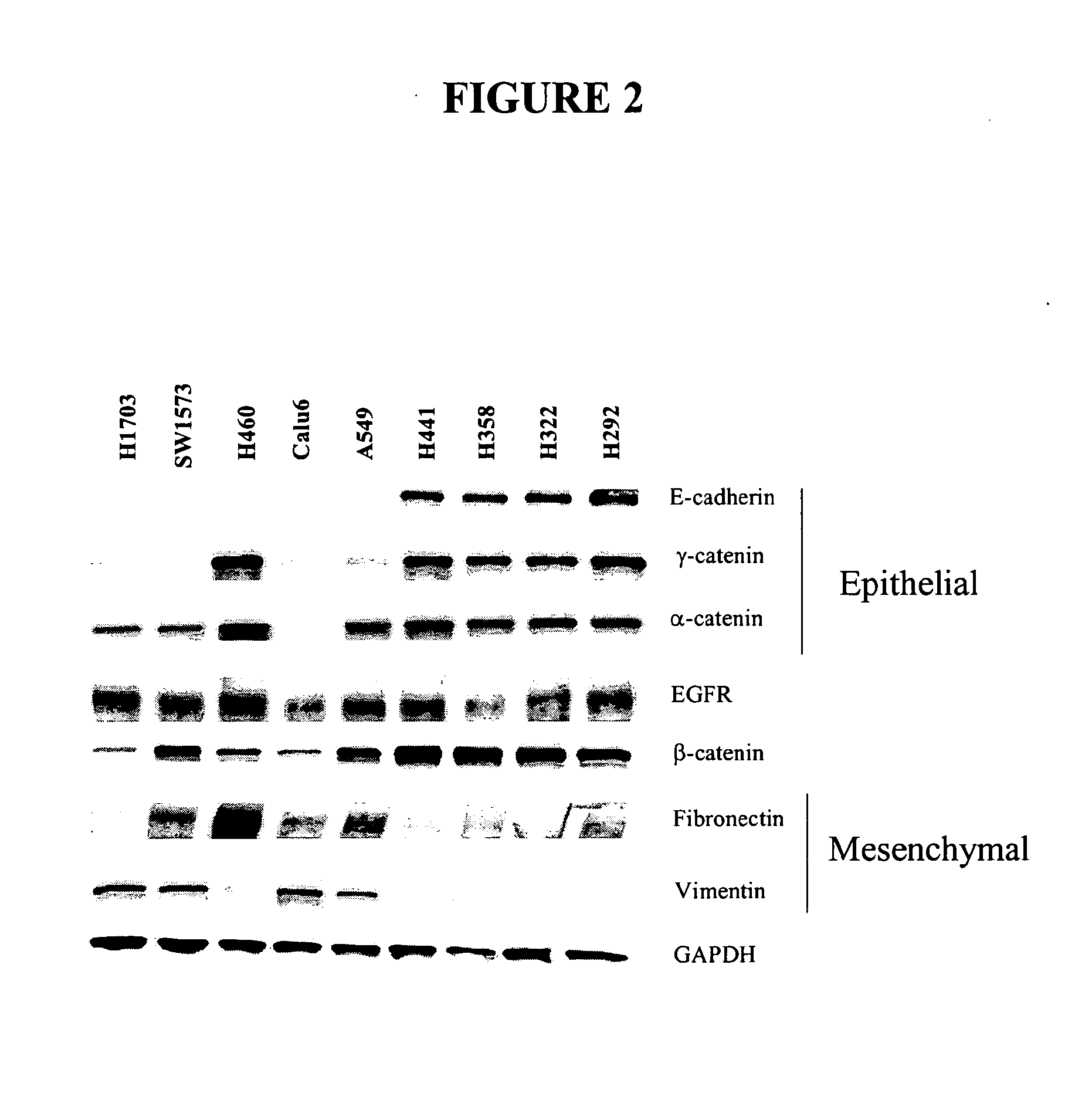
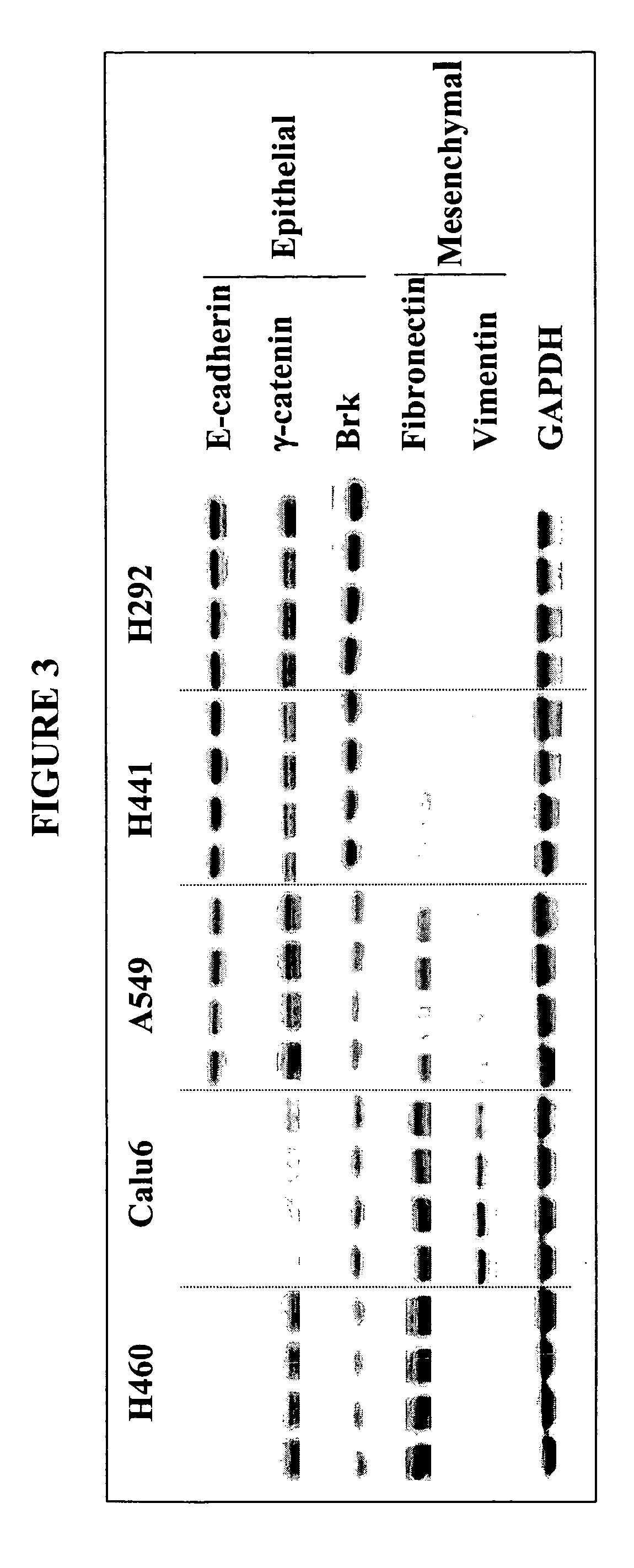
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided. pErk, HER3 and pHER are also demonstrated to be effective biomarkers for predicting sensitivity of tumor cells to IGF-1R kinase inhibitors.
Owner:OSI PHARMA INC
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
ActiveUS20090093488A1High level of biomarkerReduce sensitivityBiocideNervous disorderAbnormal tissue growthCell growth
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided. pErk, HER3 and pHER are also demonstrated to be effective biomarkers for predicting sensitivity of tumor cells to IGF-1R kinase inhibitors.
Owner:OSI PHARMA INC
Antibodies against insulin-like growth factor 1 receptor and uses thereof
InactiveUS7378503B2Slow tumor growthExtension of timePeptide/protein ingredientsPeptide sourcesInsulin-like growth factorMedicine
Antibodies which bind to IGF-IR and inhibit the binding of IGF-I and IGF-II to IGF-IR are characterized. These antibodies are implicated in anti-tumor therapy.
Owner:F HOFFMANN LA ROCHE & CO AG
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell expresses certain sensitivity or resistance biomarkers, or genomic classifiers. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate this methodology are also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
InactiveUS20110275644A1Increase valueBiocideMicrobiological testing/measurementImproved methodBiomarker (petroleum)
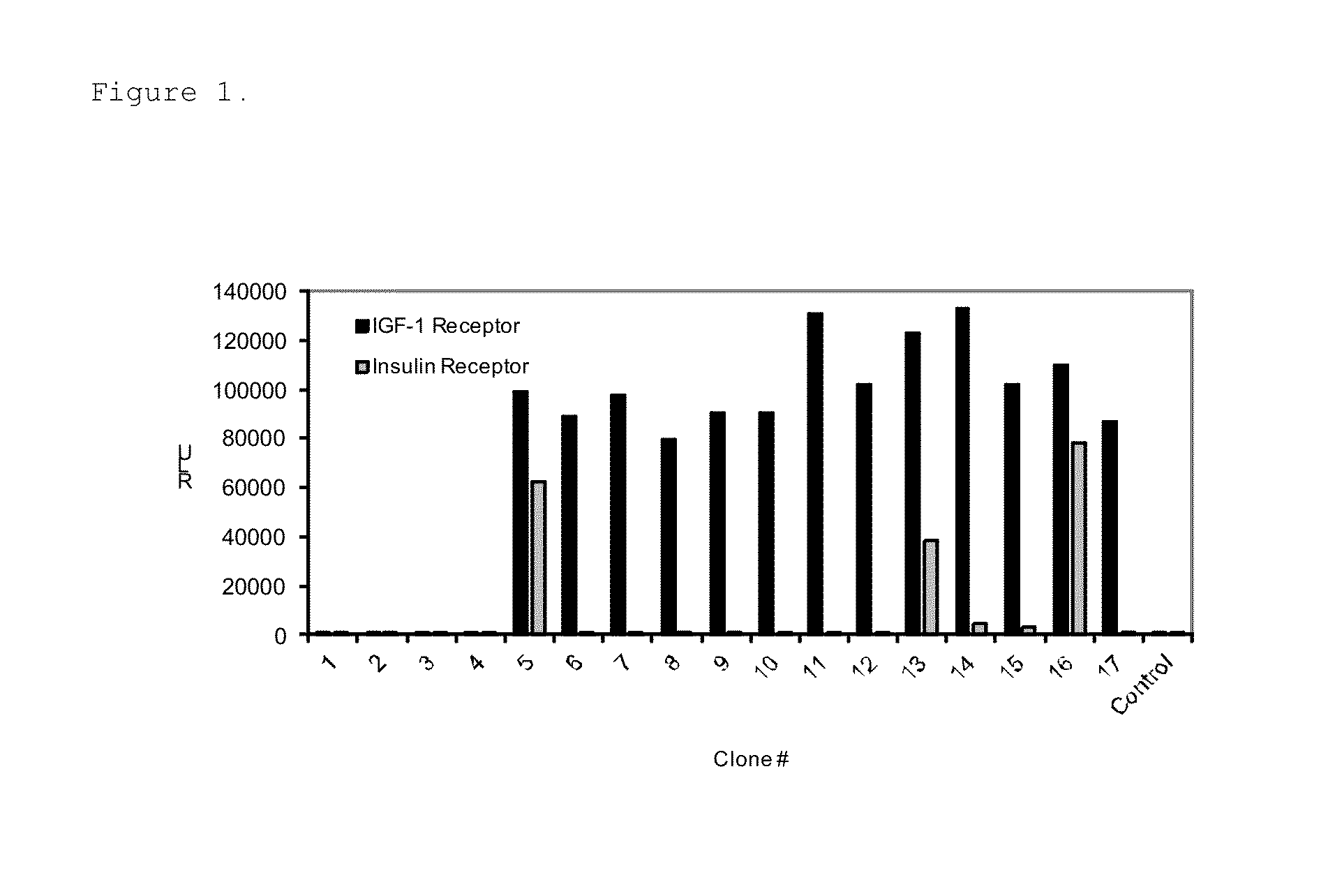
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor that inhibits both IGF-1R and IR kinases. Methods are provided for identifying patients with cancer who are likely to benefit from treatment with an IGF-1R kinase inhibitor that inhibits both IGF-1R and IR kinases. Methods are also provided for identifying patients with cancer who are likely to benefit from treatment with an IGF-1R kinase inhibitor that inhibits both IGF-1R and IR kinases, but who would likely not respond to therapy with an anti-IGF-1R antibody. Methods are also provided for identifying patients with cancer who are more likely to benefit from treatment with anti-IGF-1R antibody. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate these methods are also provided.
Owner:OSI PHARMA INC
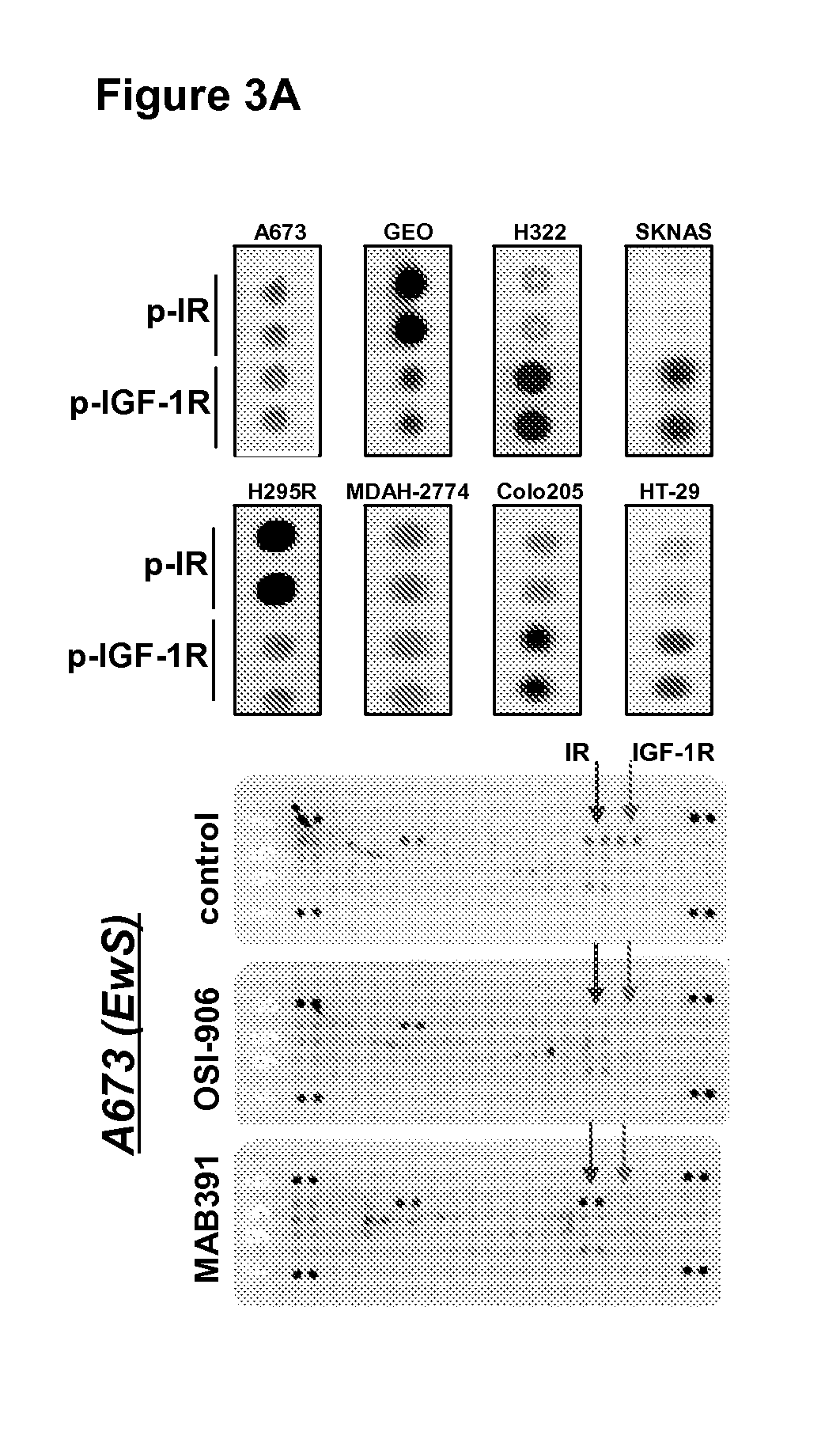
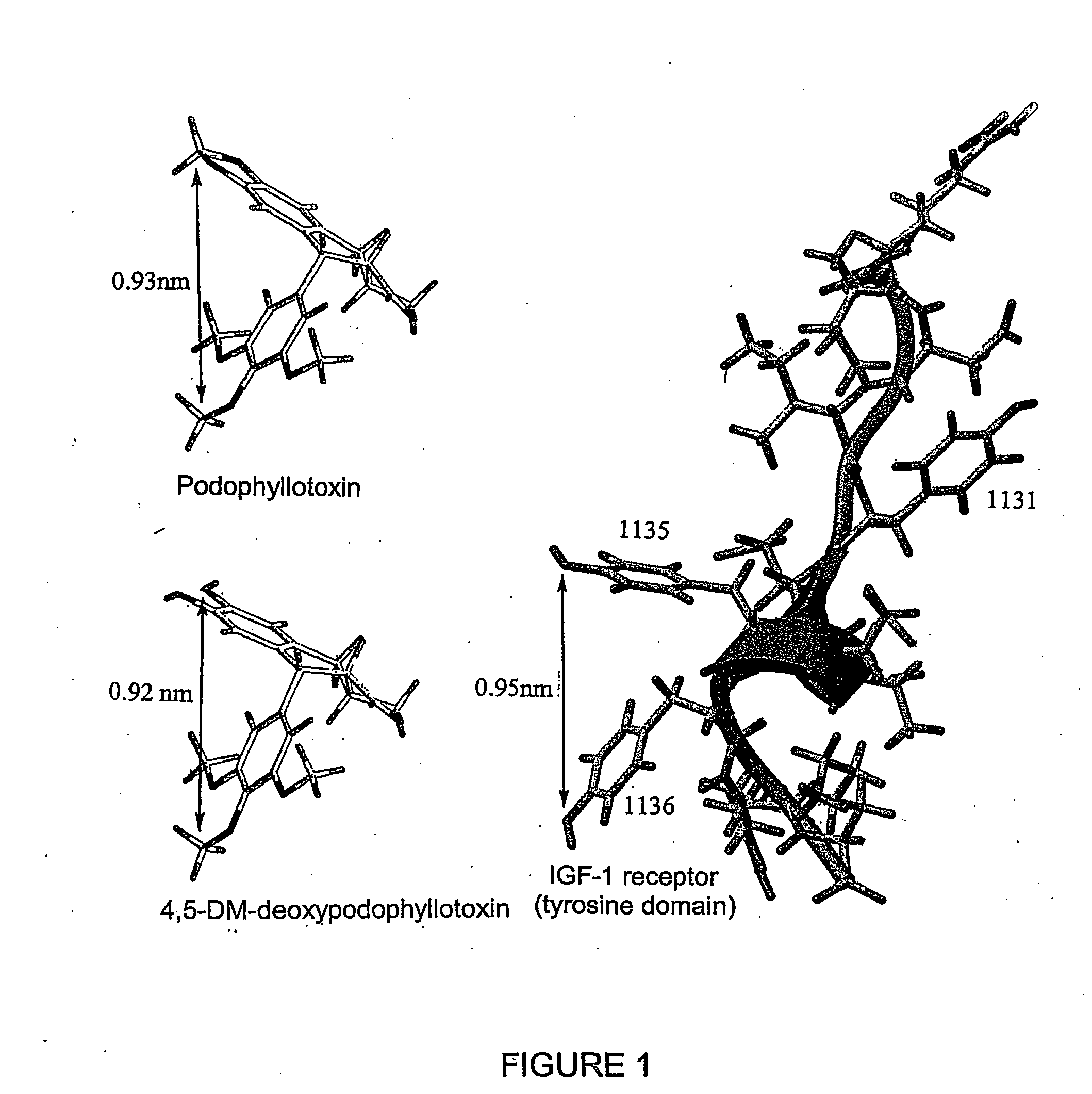
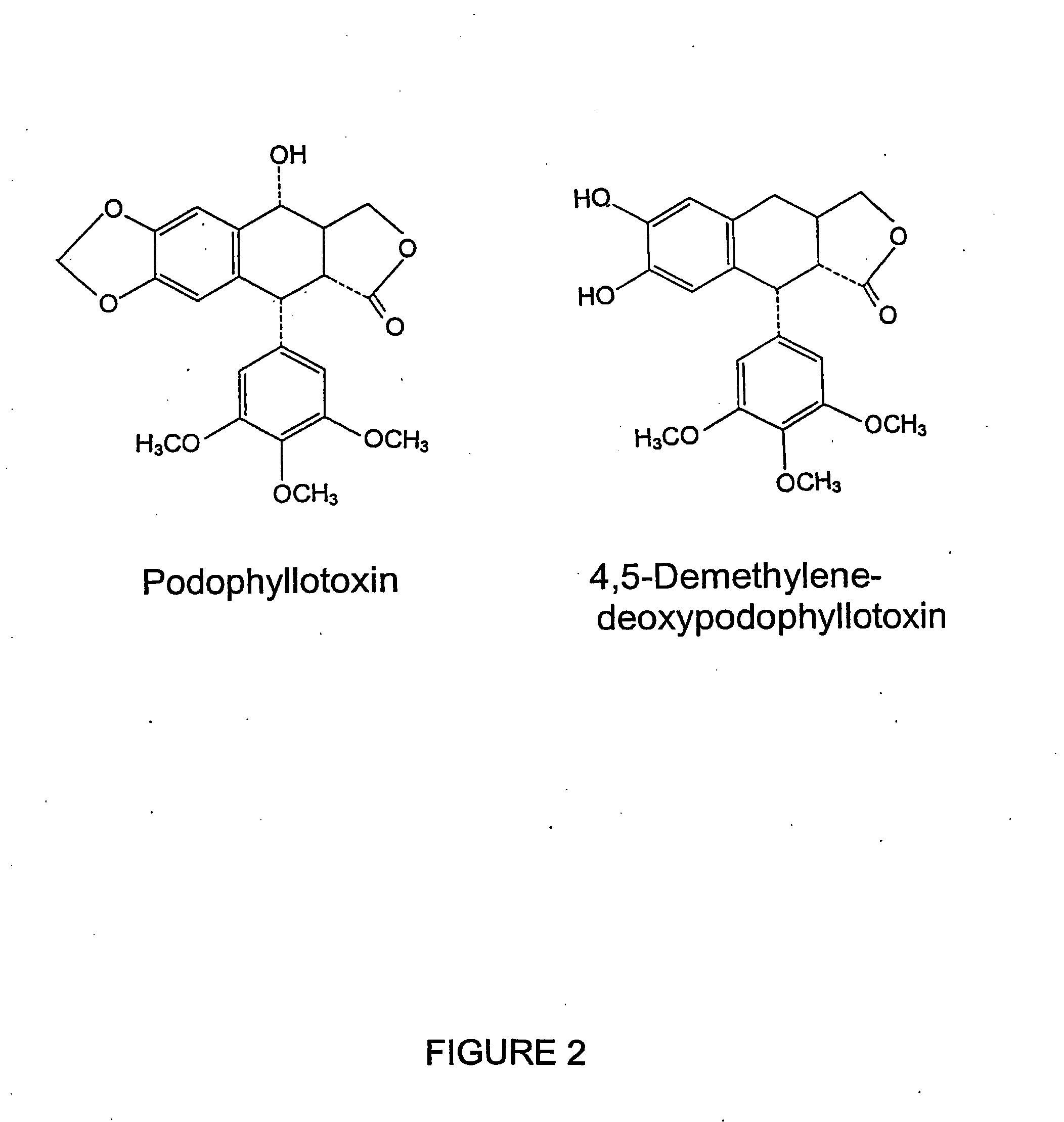
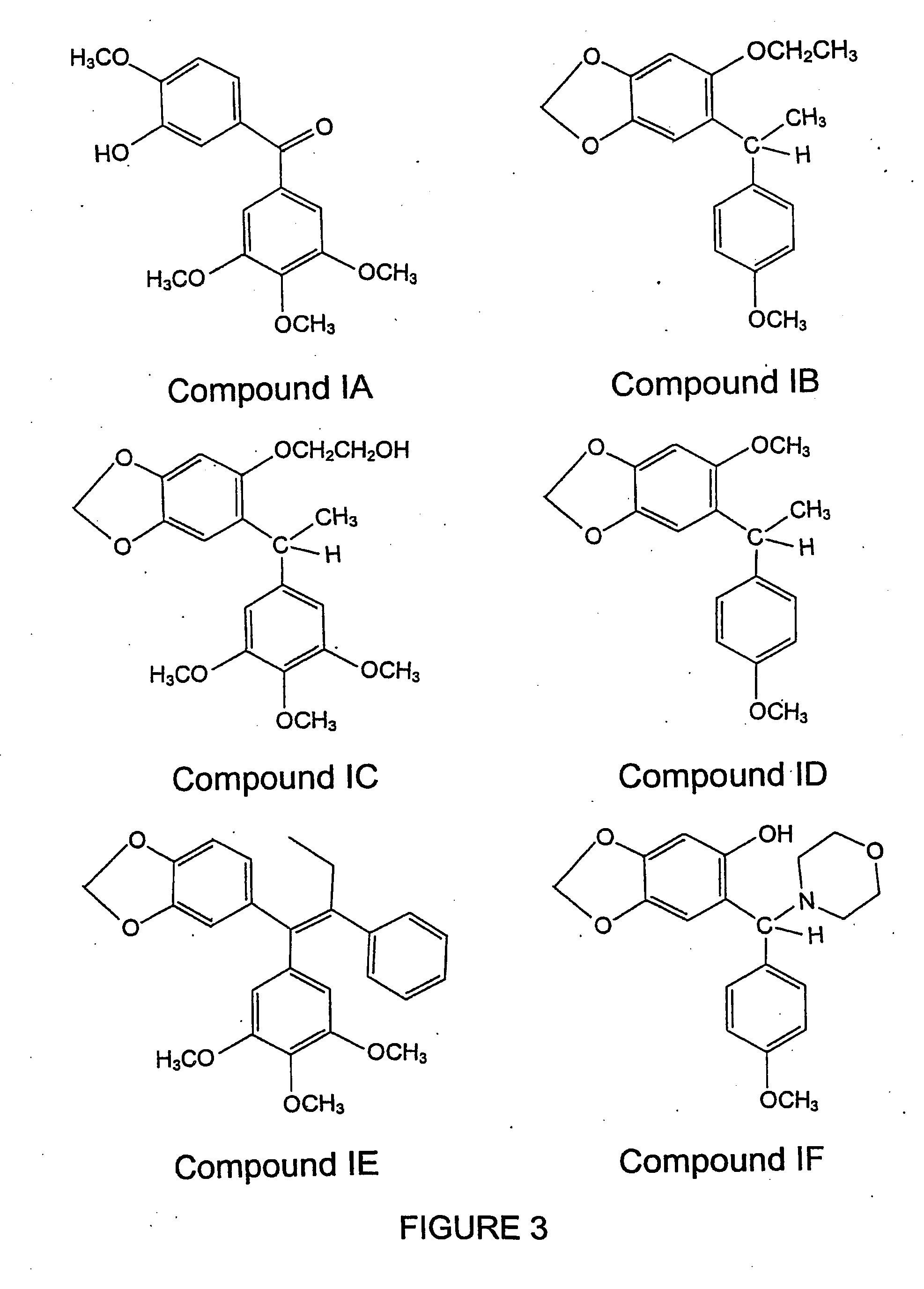
Podophyllotoxin derivatives as igf-1r inhibitors
The invention refers to new compounds, e.g. podophyllotoxin derivatives, as well as to the use thereof and of known compounds as specific inhibitors of the insulin-like growth factor-1 receptor (IGF-1R). Said compounds can be used for treatment of IGF-1 / IGF-1R dependent diseases, such as cancer, psoriasis, arteriosclerosis, certain endocrine and metabolic disorders etc.
Owner:AXELAR
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided. pErk, HER3 and pHER are also demonstrated to be effective biomarkers for predicting sensitivity of tumor cells to IGF-1R kinase inhibitors.
Owner:OSI PHARMA INC
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
InactiveUS20120201832A1Microbiological testing/measurementAntibody ingredientsCell growthImproved method
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell expresses certain sensitivity or resistance biomarkers, or genomic classifiers. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate this methodology are also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
Biological markers predictive of Anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors in hepatocellular carcinoma
InactiveUS20120214830A1Improve the level ofHigh sensitivityBiocideOrganic active ingredientsAbnormal tissue growthHepatocellular carcinoma
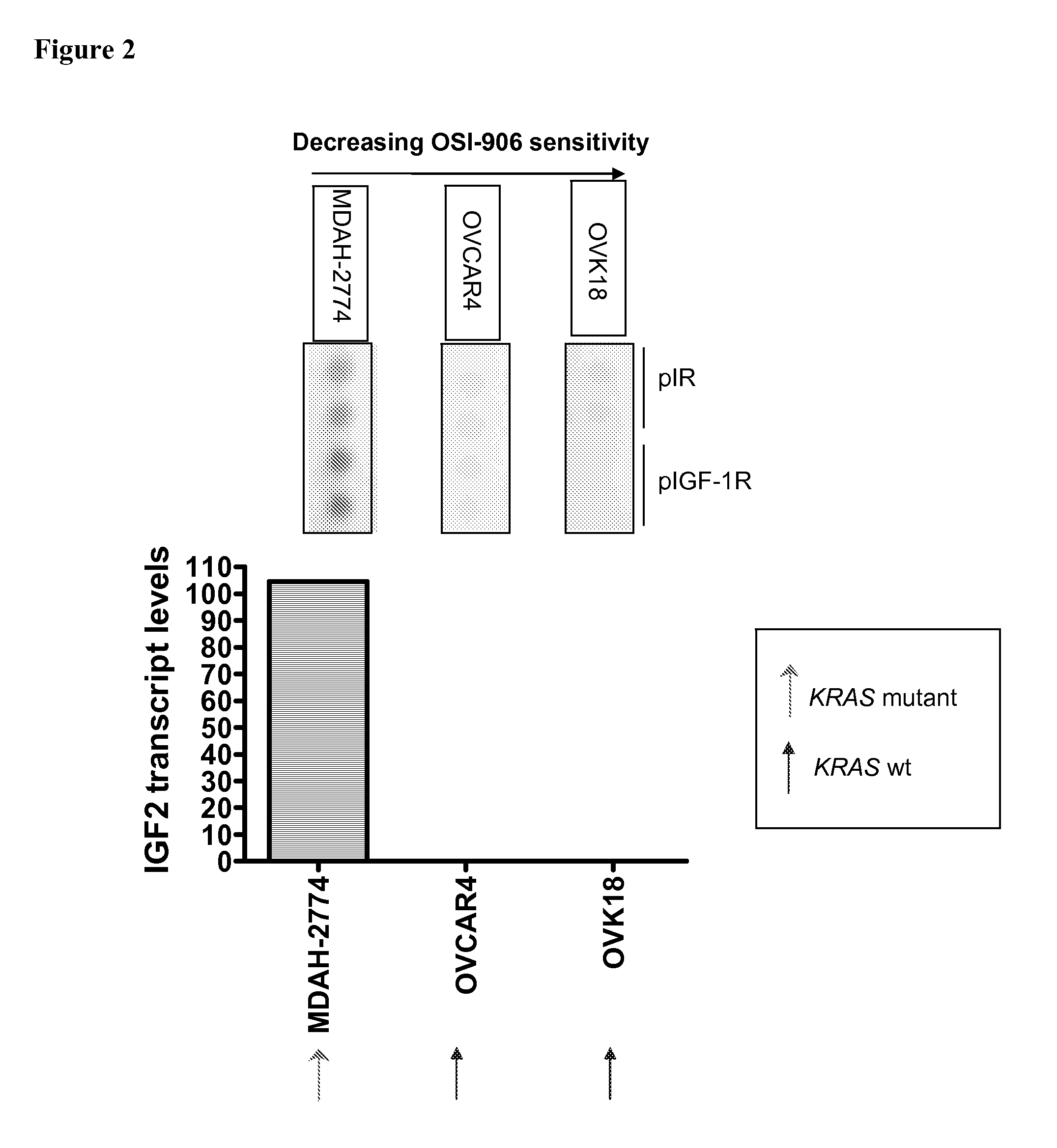
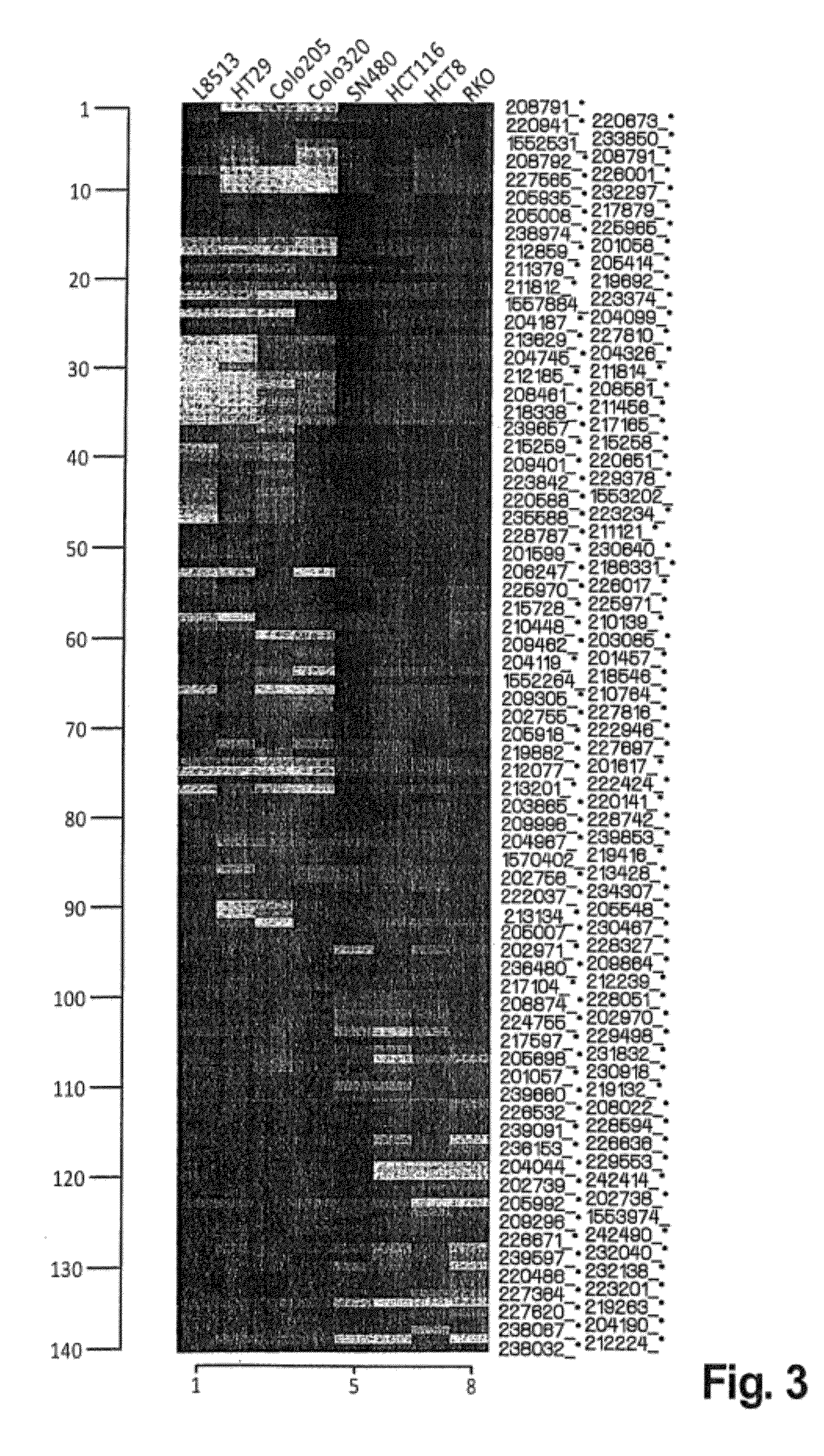
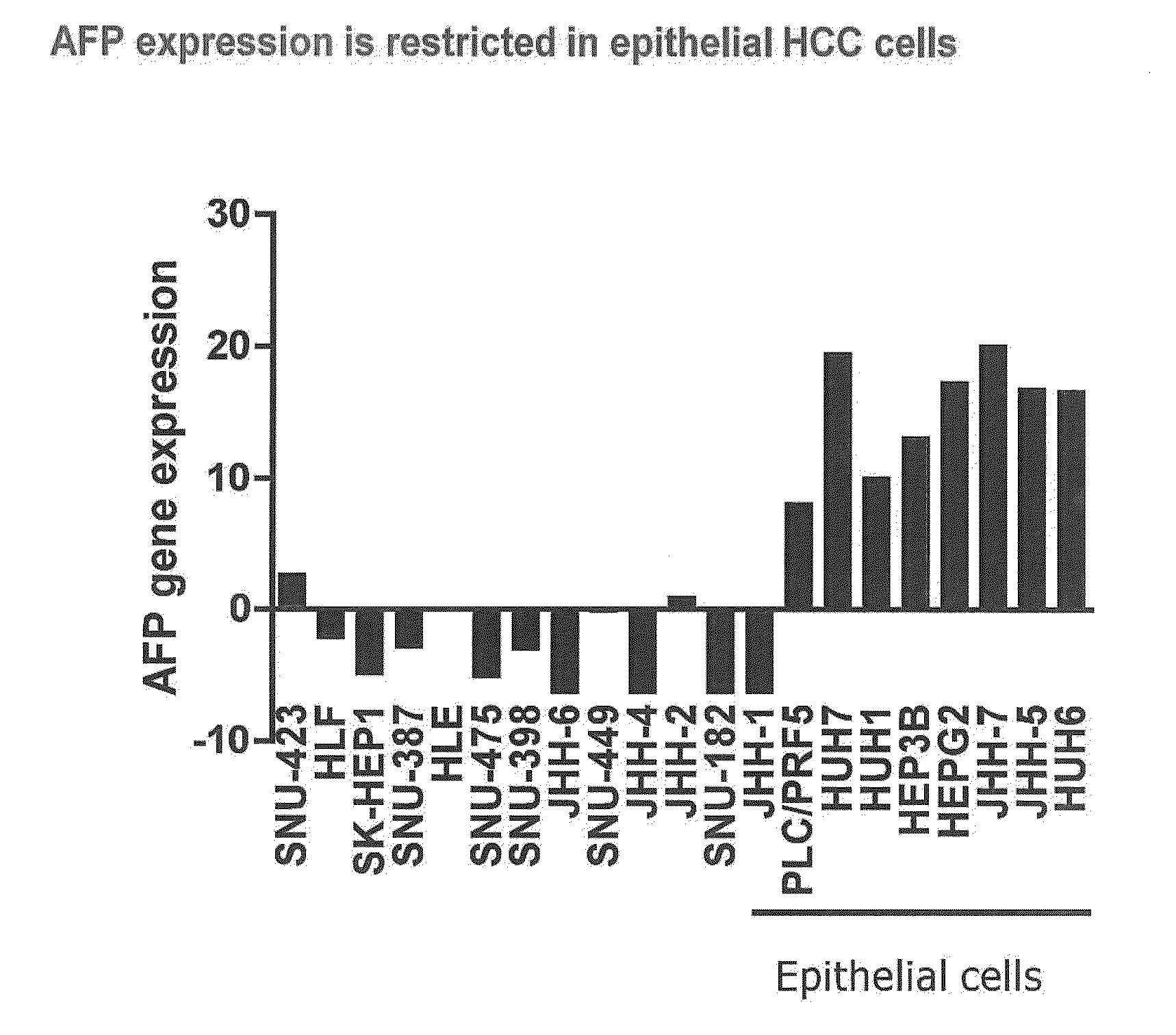
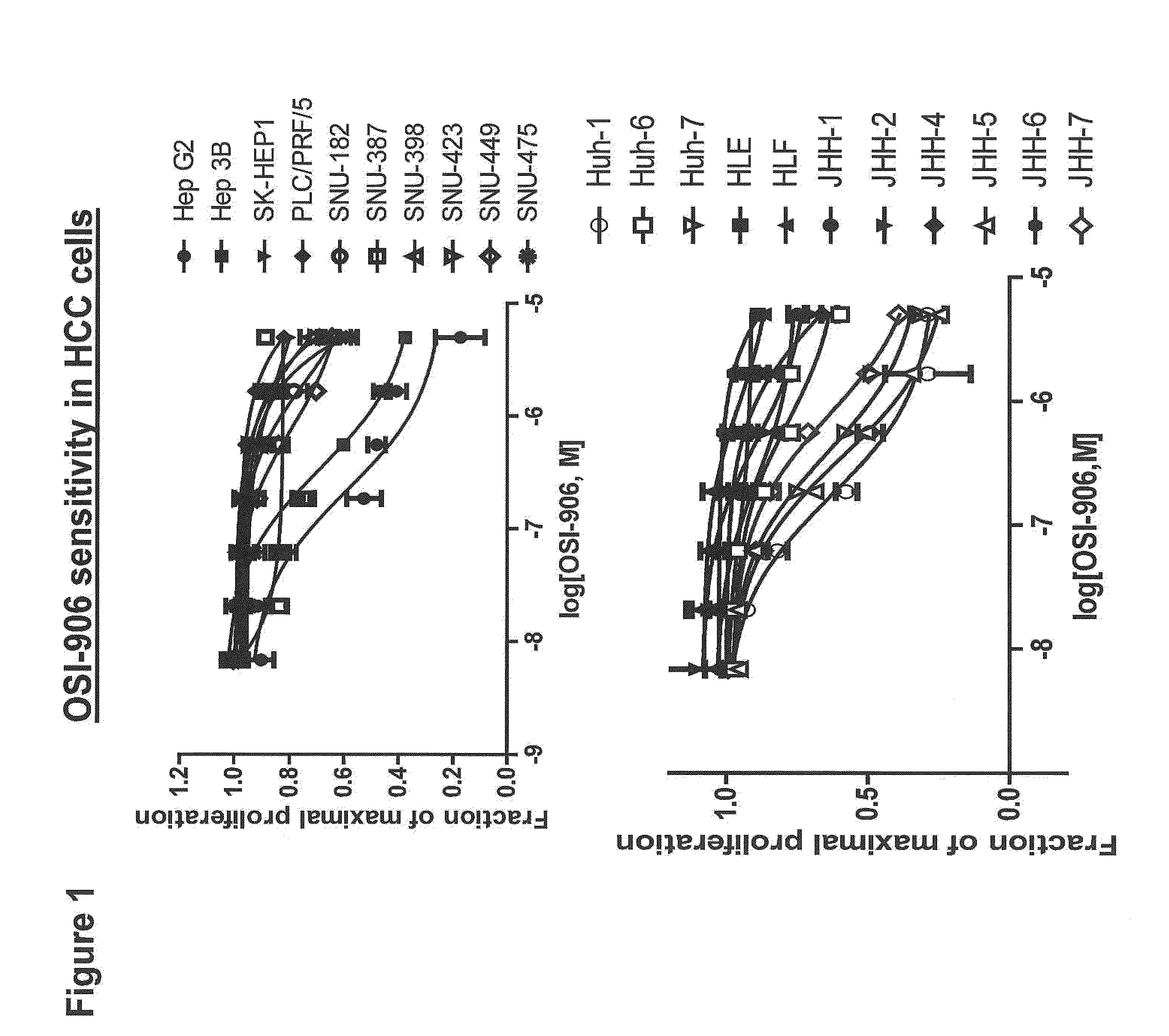
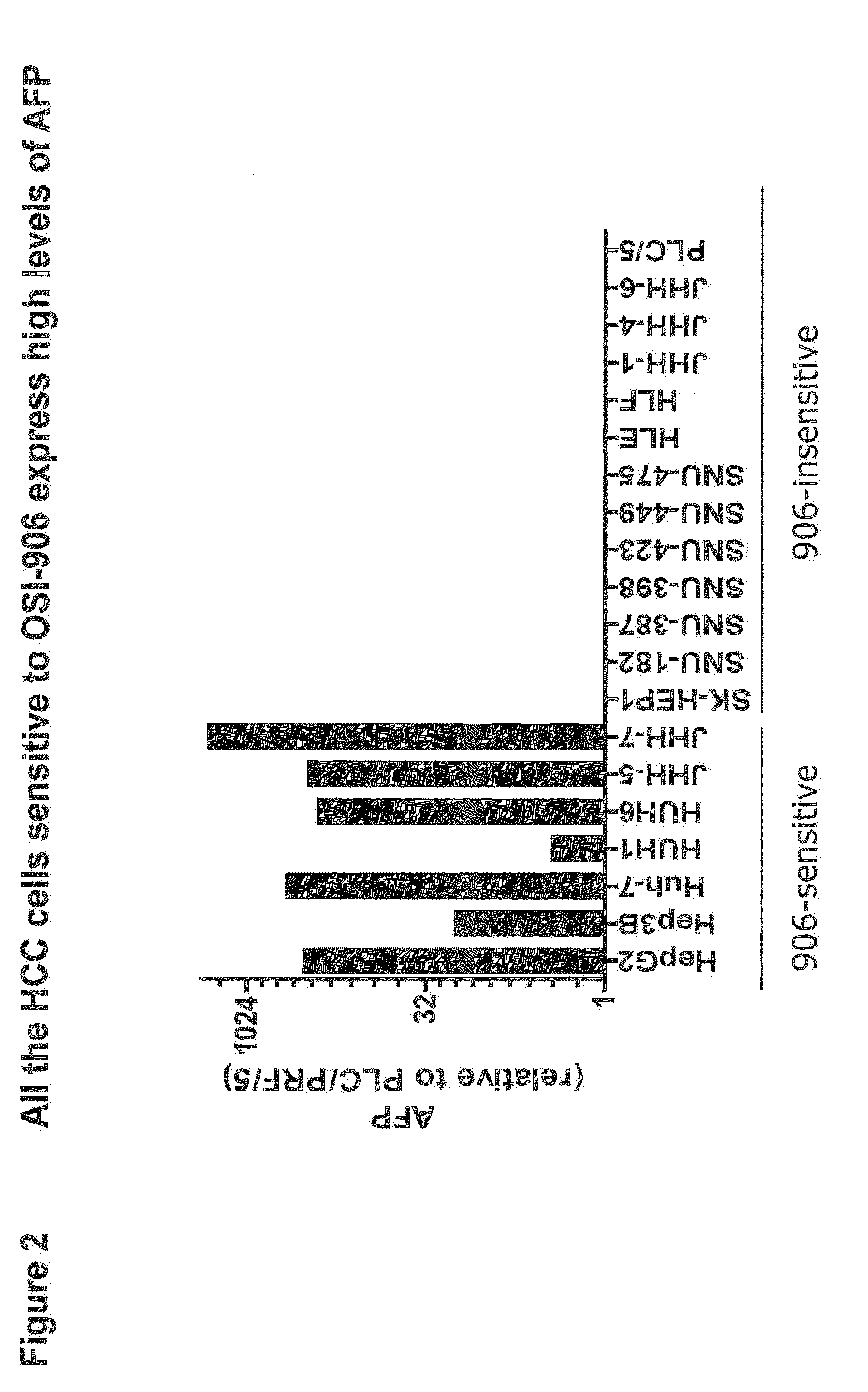
The present invention provides diagnostic methods for predicting the effectiveness of treatment of an hepatocellular carcinoma (HCC) patient with an IGF-1R kinase inhibitor by assessing whether the HCC tumor cells express a high level of AFP, or whether serum levels of AFP protein are high. The present invention also provides diagnostic methods for predicting the effectiveness of treatment of cancer patients with IGF-1R kinase inhibitors, based on a determination of the expression level of IR, IGF-2, IGFBP3 or IGFBP7 in tumor cells, or a 4-gene index calculated using the expression vales for each of these four genes, which can be used to identify tumors that will be sensitive to IGF-1R kinase inhibitors, and also those that will be insensitive. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methods are also provided.
Owner:OSI PHARMA LLC
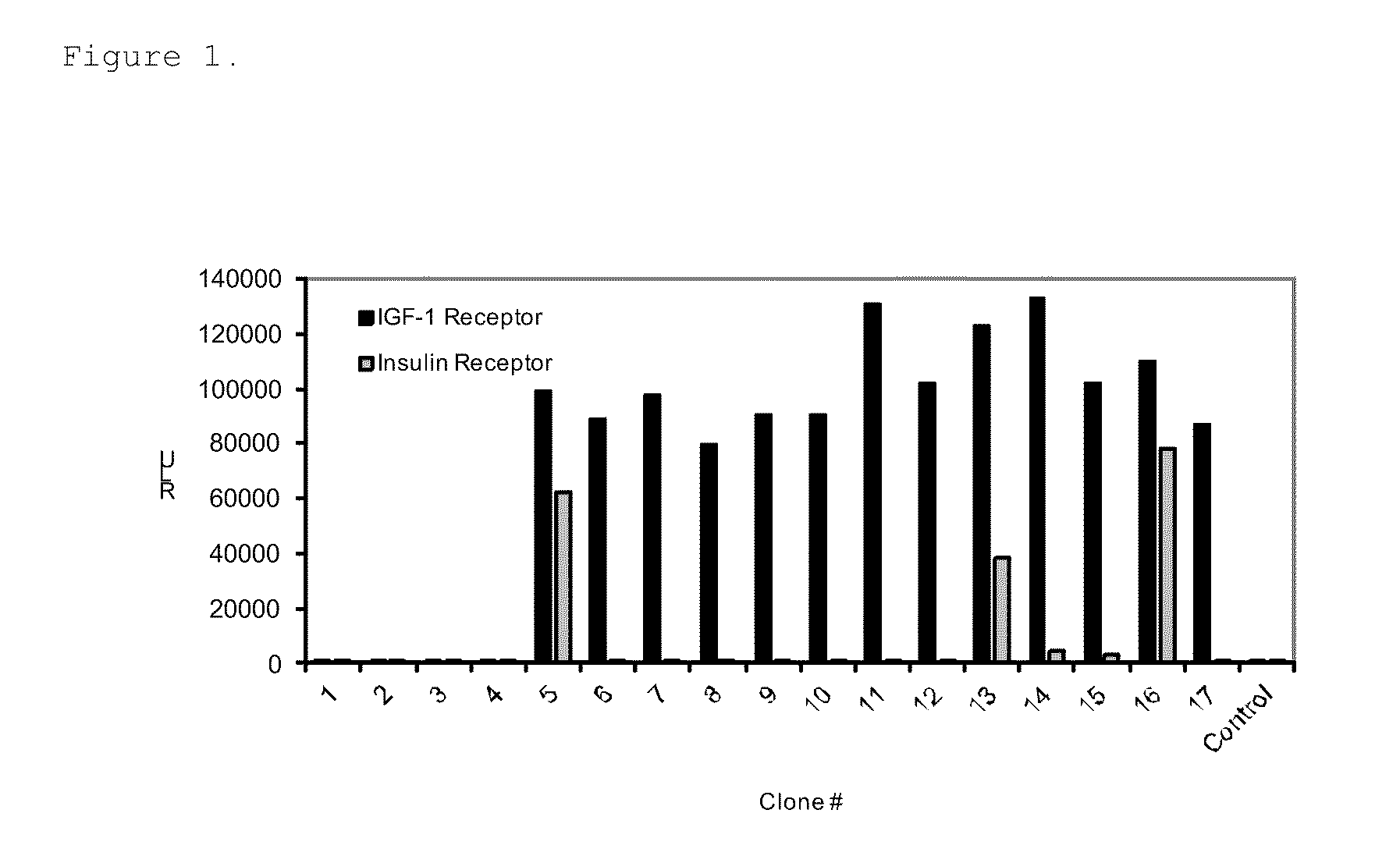
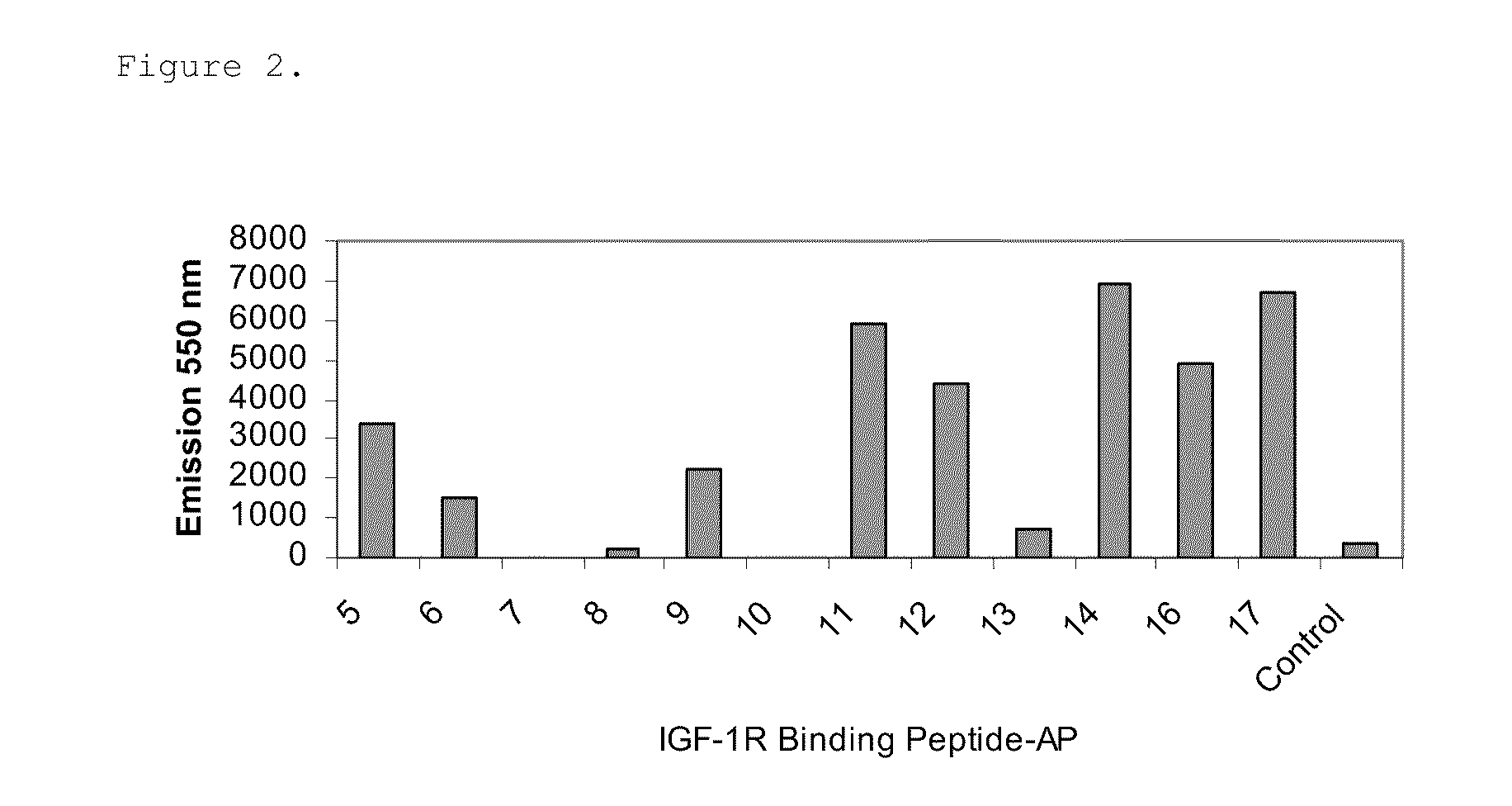
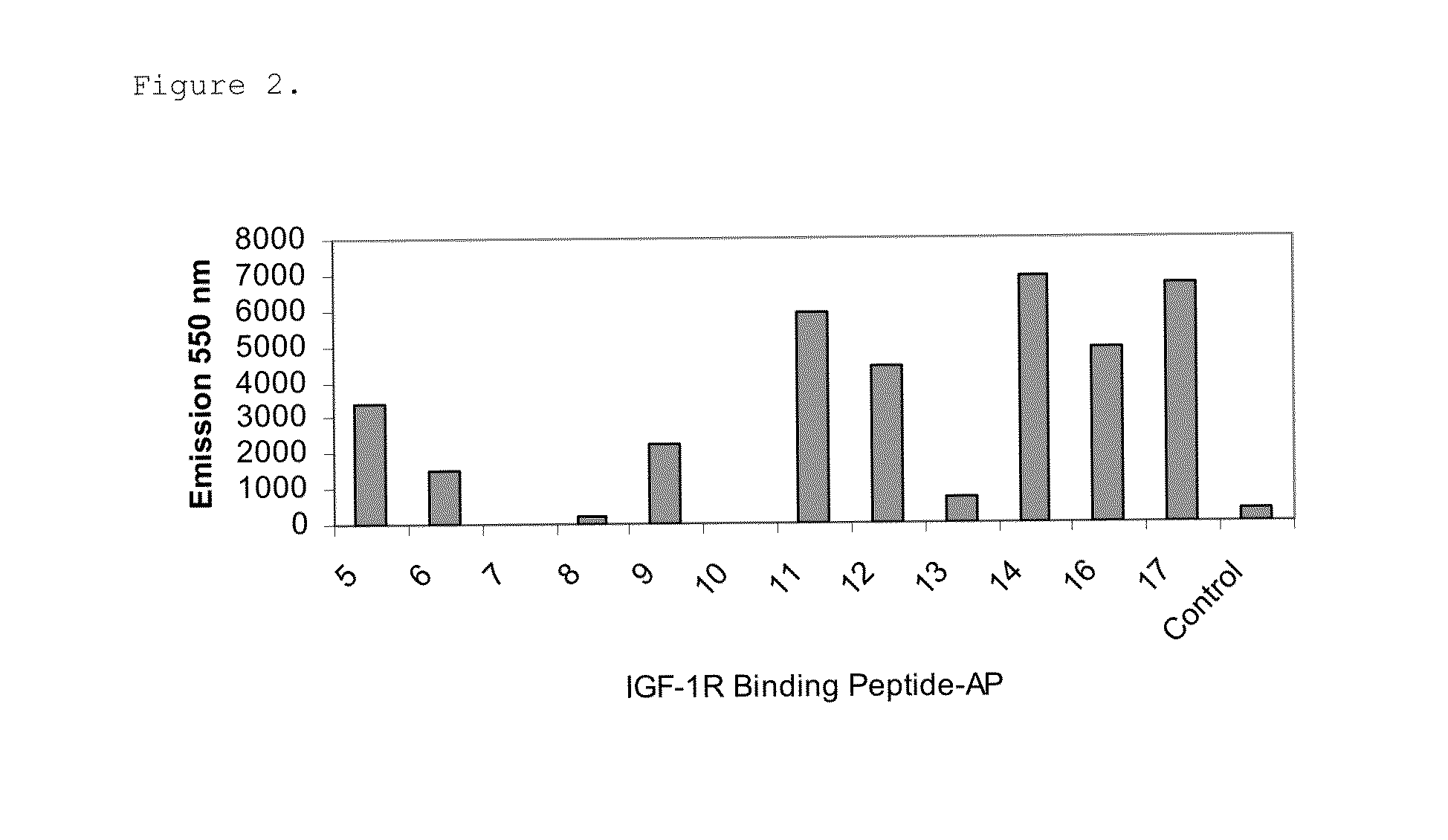
Insulin-like growth factor 1 receptor binding peptides
The present invention relates to insulin-like growth factor 1 receptor binding peptides, polynucleotides encoding them, and methods of making and using the foregoing.
Owner:JANSSEN BIOTECH INC
Methods and compositions for treating cancers using antisense
ActiveUS20180201939A1Prevent regenerationOptimal anti-tumor responseOrganic active ingredientsSpecial deliveryCancer cellGlioblastoma
The present disclosure relates to compositions and methods for treating cancers using antisense (AS) nucleic acids directed against Insulin-like Growth Factor 1 Receptor (IGF-1R). The AS may be administered to the patients systemically, or may be used to produce an autologous cancer cell vaccine. In embodiments, the AS are provided in an implantable irradiated biodiffusion chamber comprising tumor cells and an effective amount of the AS. The chambers are irradiated and implanted in the abdomen of subjects and stimulate an immune response that attacks tumors distally. The compositions and methods disclosed herein may be used to treat many different kinds of cancer, for example glioblastoma.
Owner:THOMAS JEFFERSON UNIV
Insulin-like growth factor 1 receptor -specific antibodies and uses thereof
ActiveUS20170015748A1Prolonged Circulatory Half-LifeEfficient transportGeneral/multifunctional contrast agentsOmicsAntigen bindingBiology
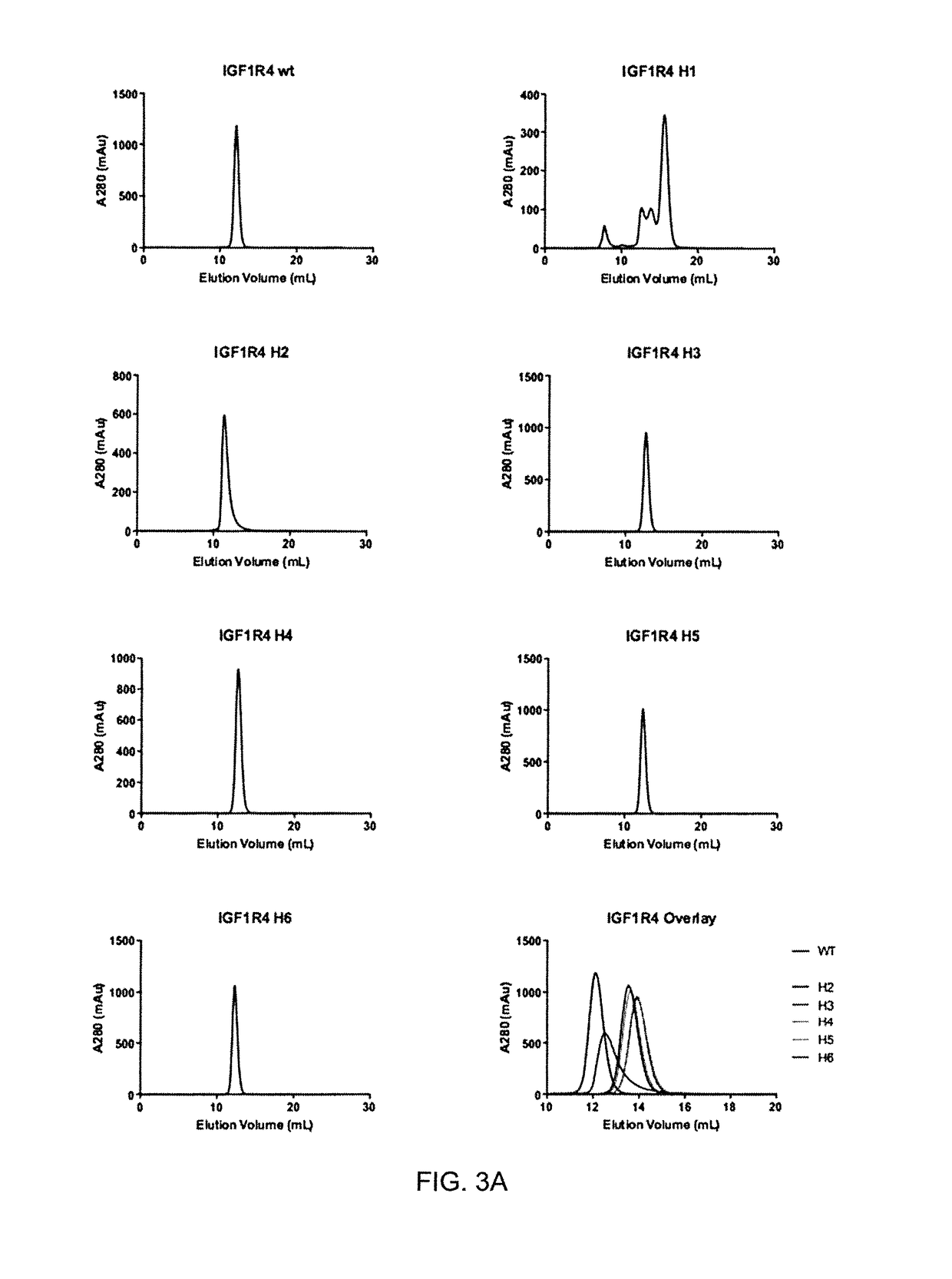
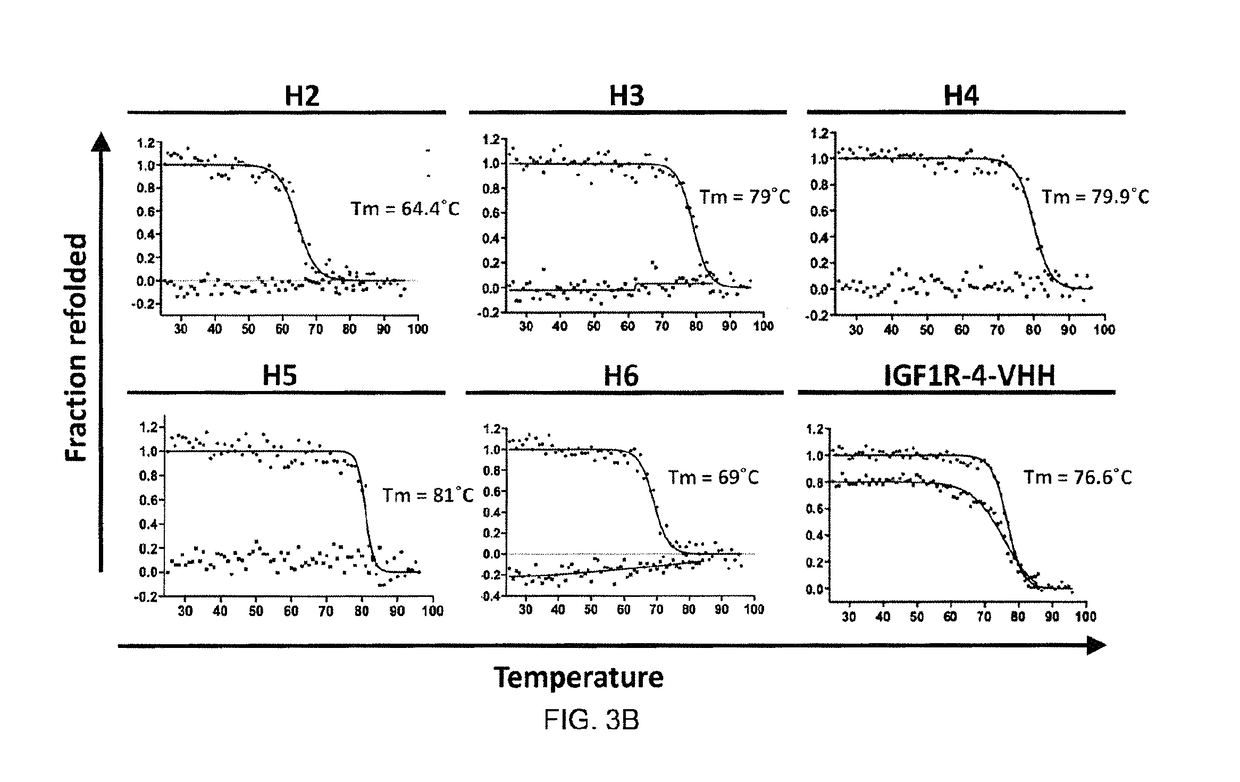
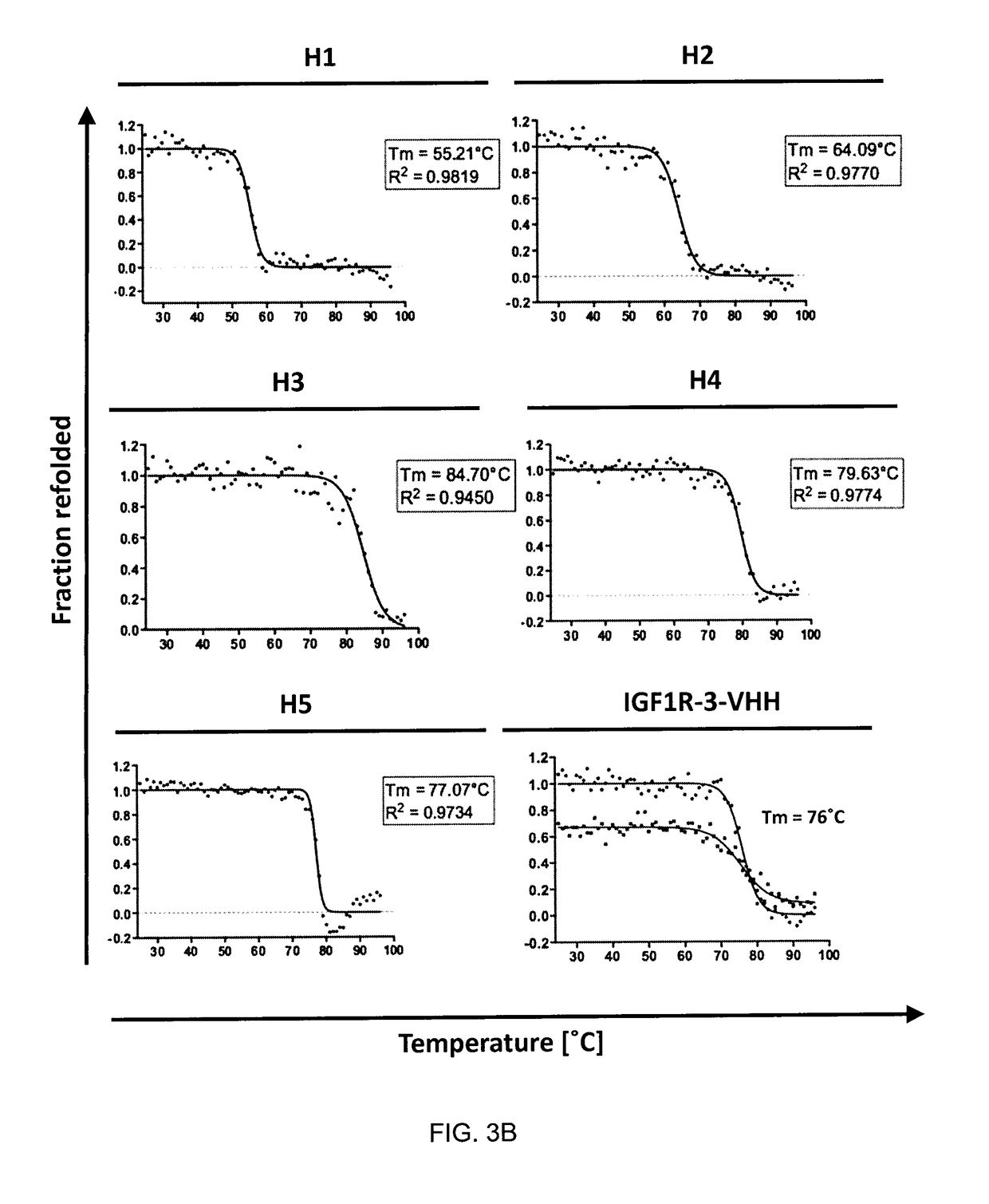
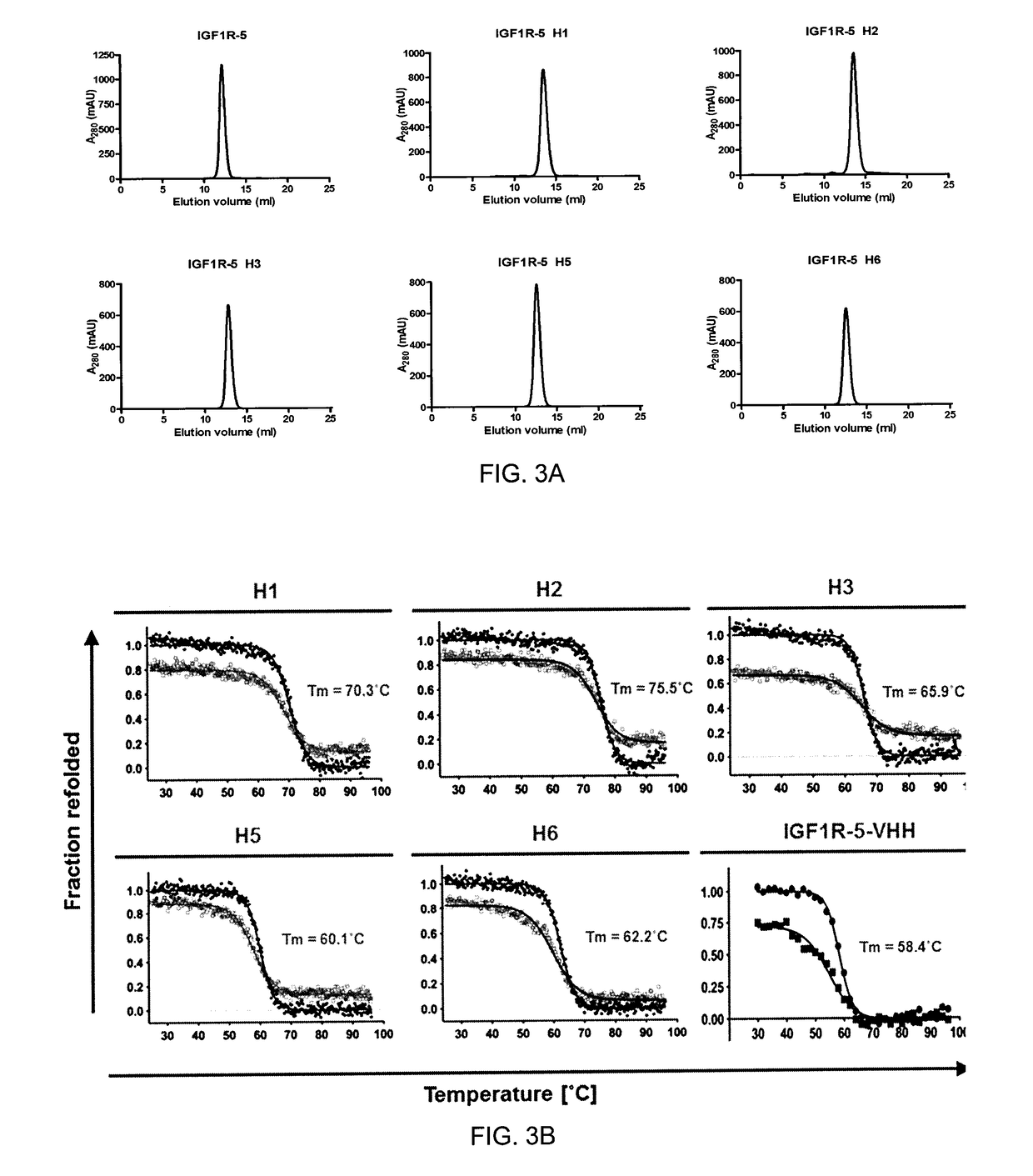
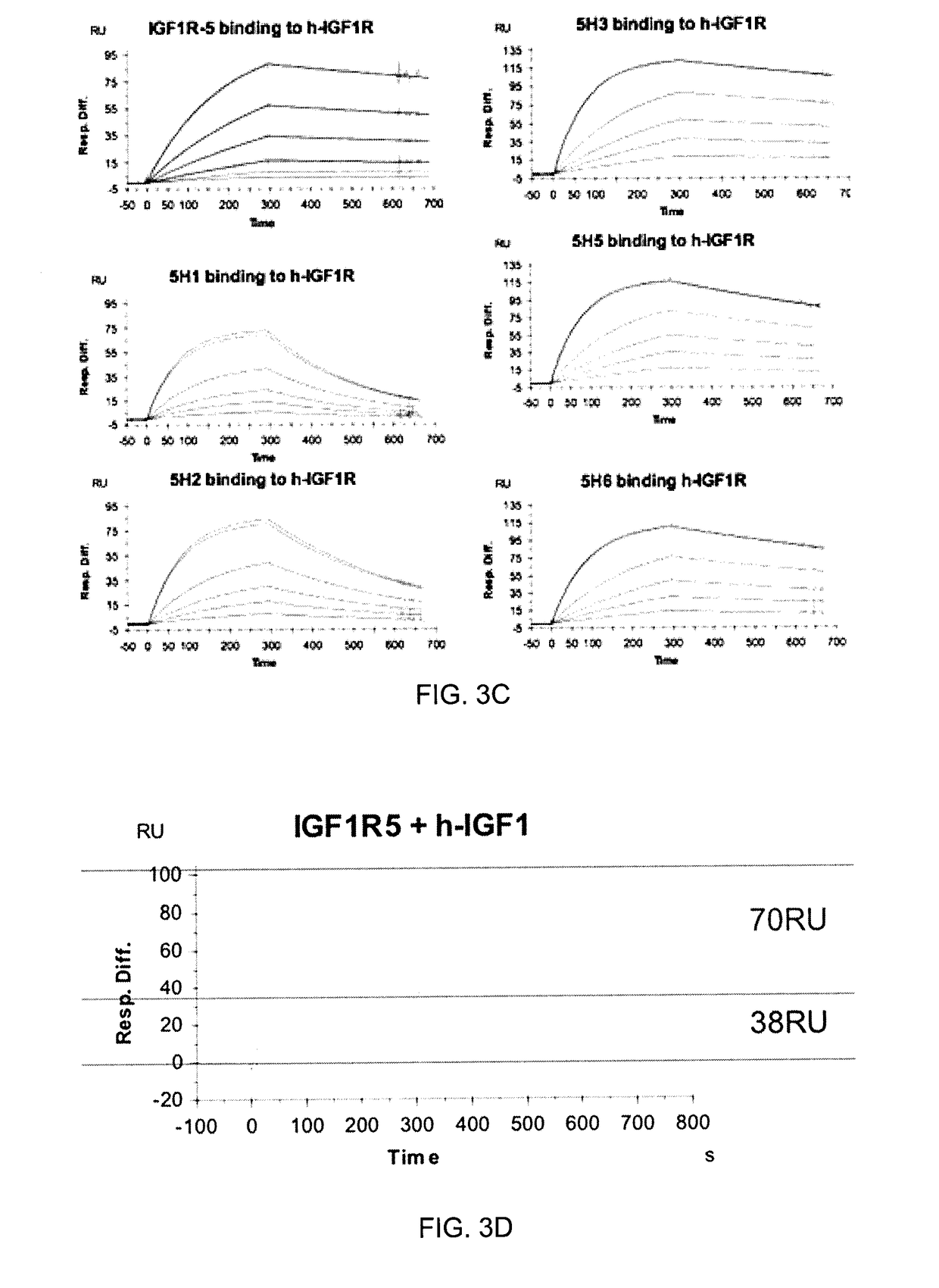
The blood-brain barrier (BBB) prevents transport of molecules larger than 500 Dal tons from blood to brain. Receptor-mediated transcytosis (RMT) facilitates transport across the BBB of specific molecules that bind receptors on brain endothelial cells that form the BBB. An insulin-like growth factor 1 receptor (IGF 1R)-binding antibody or fragment thereof is identified that transmigrates the BBB by RMT. The antibody or fragment is used to deliver a cargo molecule across the BBB, wherein the cargo molecule may be a therapeutic or detectable agent. The antibody is a camelid VHH, prepared by immunizing a llama with a 933-amino acid IGF 1R polypeptide. Humanized forms of the camelid VHH are also generated.
Owner:NAT RES COUNCIL OF CANADA
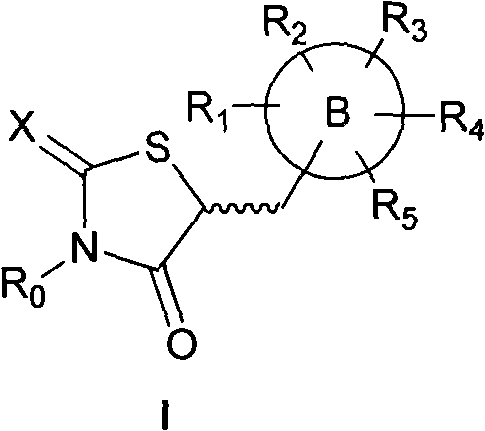
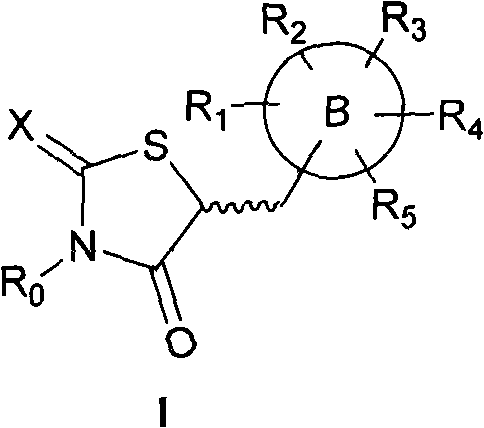
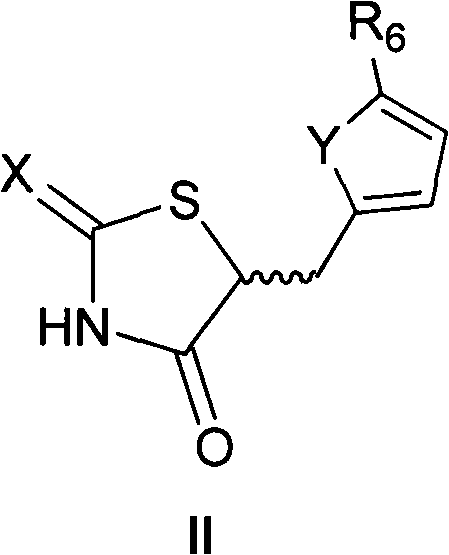
Application of 5-substituted-2,4-thiazolidinedione compound in preparation of IGF1R (insulin-like growth factor 1 receptor) function regulating medicaments
InactiveCN102078318AGrowth inhibitionPrevent proliferationOrganic active ingredientsAntineoplastic agentsDiseaseInsulin-like growth factor
The invention relates to an application of a 5-substituted-2,4-thiazolidinedione compound as shown in general formula (I) disclosed in the specification or pharmaceutically acceptable salts thereof in the preparation of IGF1R (insulin-like growth factor 1 receptor) function regulating medicaments. The 5-substituted-2,4-thiazolidinedione compound has a better effect in the aspect of inhibiting IGF1R kinase activity, and the IGF1R plays an important role in promoting the proliferation and differentiation of tumor cells, so that the compound provided by the invention can be used for preparing the medicaments for treating tumor diseases.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Insulin-like growth factor 1 receptor-specific antibodies and uses thereof
ActiveUS10112998B2Efficient transportEasy to detectHybrid immunoglobulinsNervous disorderInsulin-like growth factorCereblon
The blood-brain barrier (BBB) prevents transport of molecules larger than 500 Dal tons from blood to brain. Receptor-mediated transcytosis (RMT) facilitates transport across the BBB of specific molecules that bind receptors on brain endothelial cells that form the BBB. An insulin-like growth factor 1 receptor (IGF 1R)-binding antibody or fragment thereof is identified that transmigrates the BBB by RMT. The antibody or fragment is used to deliver a cargo molecule across the BBB, wherein the cargo molecule may be a therapeutic or detectable agent. The antibody is a camelid VHH, prepared by immunizing a llama with a 933-amino acid IGF 1R polypeptide. Humanized forms of the camelid VHH are also generated.
Owner:NAT RES COUNCIL OF CANADA
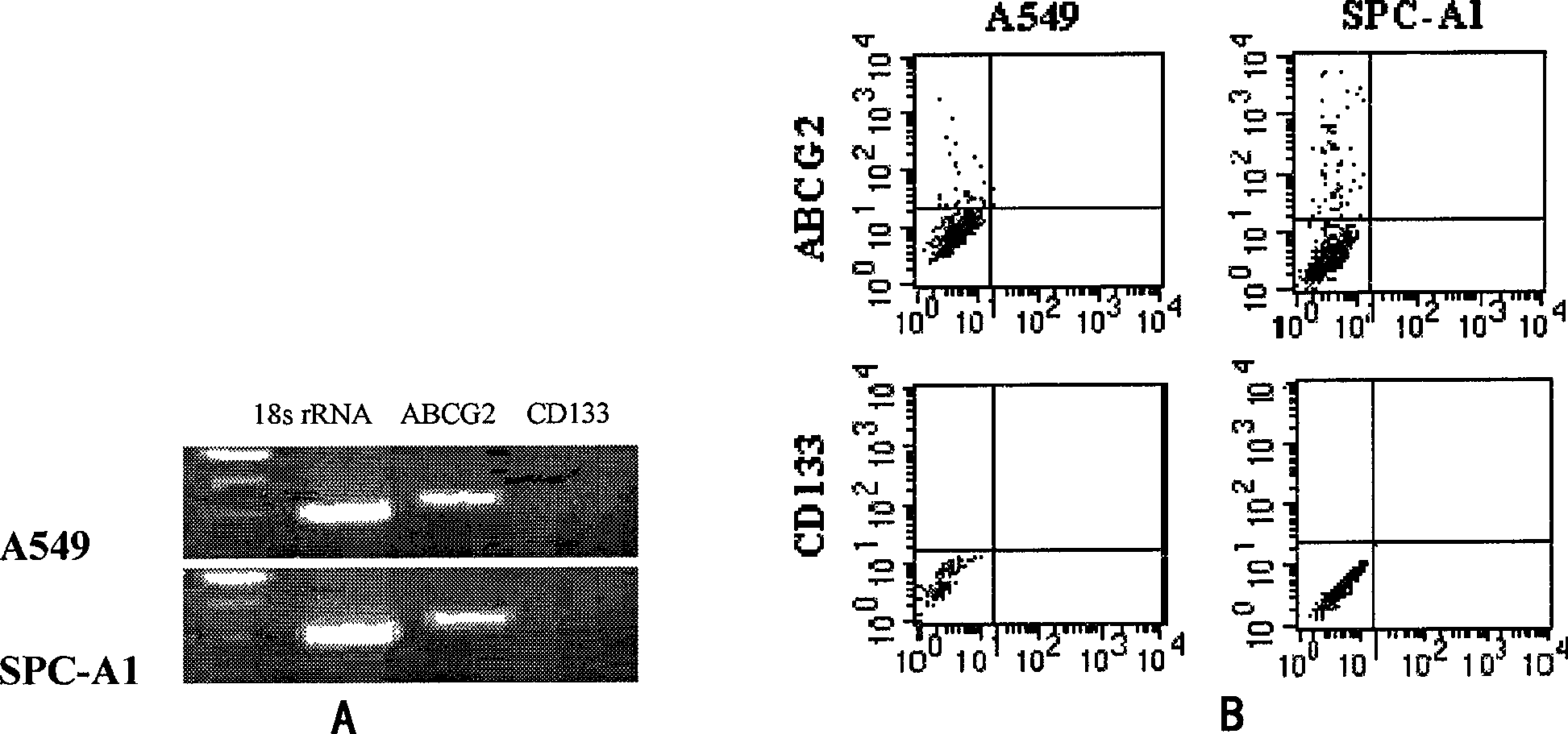
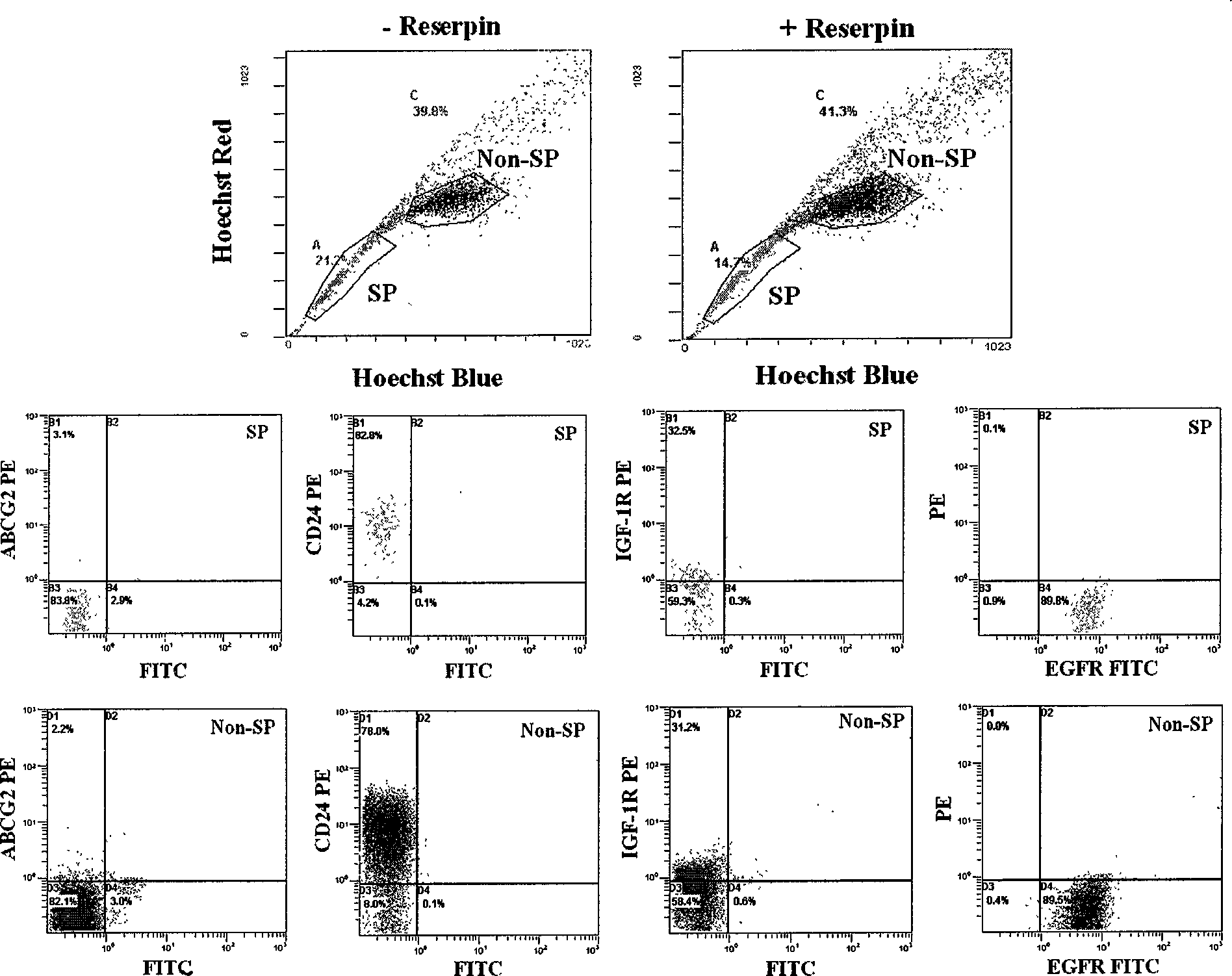
Method for isolating and identifying human lung adenocarcinoma stem cell
InactiveCN101463342AMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCD24Cancer research
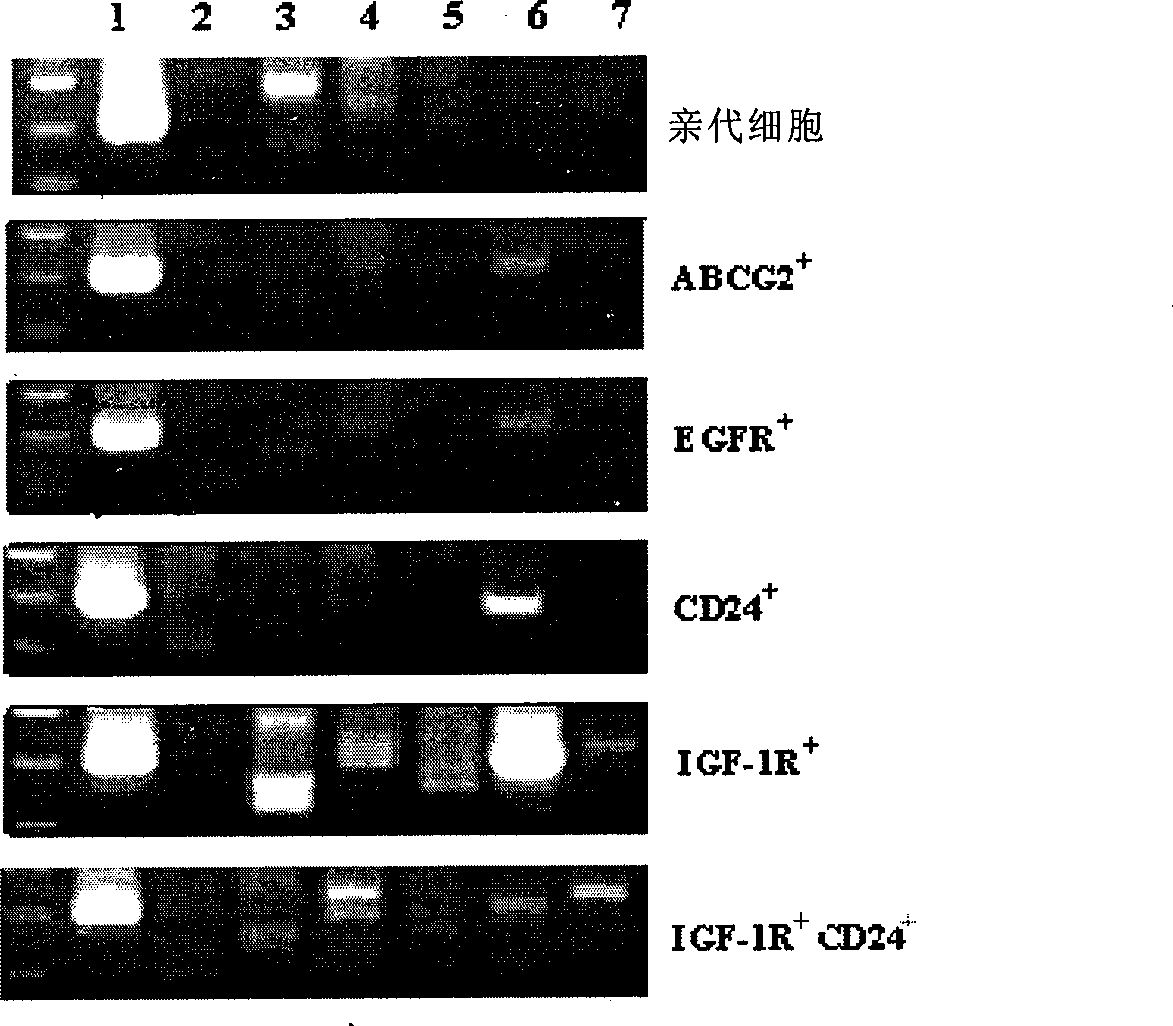
The invention belongs to the field of cytobiology, and discloses a method for separating or identifying lung adenocarcinoma stem cells (group) from lung adenocarcinoma cells (group); the invention also discloses a reagent and a kit for separating or identifying lung adenocarcinoma stem cells (group). The invention shows that the lung adenocarcinoma stem cells have special mark CD24 and insulin-like growth factor-1 receptor (IGF-1R) for the first time, and provides efficient path for separating or identifying lung adenocarcinoma stem cells (group).
Owner:SHANGHAI INST OF ONCOLOGY
Insulin-like growth factor 1 receptor -specific antibodies and uses thereof
ActiveUS20170015749A1Prolonged Circulatory Half-LifeEfficient transportHybrid immunoglobulinsNervous disorderInsulin-like growth factorAntigen binding
The blood-brain barrier (BBB) prevents transport of molecules larger than 500 Daltons from blood to brain. Receptor-mediated transcytosis (RMT) facilitates transport across the BBB of specific molecules that bind receptors on brain endothelial cells that form the BBB. An insulin-like growth factor 1 receptor (IGF1R)-binding antibody or fragment thereof is identified that transmigrates the BBB by RMT. The antibody or fragment is used to deliver a cargo molecule across the BBB, wherein the cargo molecule may be a therapeutic or detectable agent. The antibody is a camelid VHH, prepared by immunizing a llama with a 933-amino acid IGF1R polypeptide. Humanized forms of the camelid VHH are also generated.
Owner:NAT RES COUNCIL OF CANADA
Insulin-like growth factor 1 receptor -specific antibodies and uses thereof
ActiveUS20170022277A1Prolonged Circulatory Half-LifeEfficient transportNervous disorderGeneral/multifunctional contrast agentsInsulin-like growth factorAntigen binding
The blood-brain barrier (BBB) prevents transport of molecules larger than 500 Dal tons from blood to brain. Receptor-mediated transcytosis (RMT) facilitates transport across the BBB of specific molecules that bind receptors on brain endothelial cells that form the BBB. An insulin-like growth factor 1 receptor (IGF 1R)-binding antibody or fragment thereof is identified that transmigrates the BBB by RMT. The antibody or fragment is used to deliver a cargo molecule across the BBB, wherein the cargo molecule may be a therapeutic or detectable agent. The antibody is a camelid VHH, prepared by immunizing a llama with a 933-amino acid IGF 1R polypeptide. Humanized forms of the camelid VHH are also generated.
Owner:NAT RES COUNCIL OF CANADA
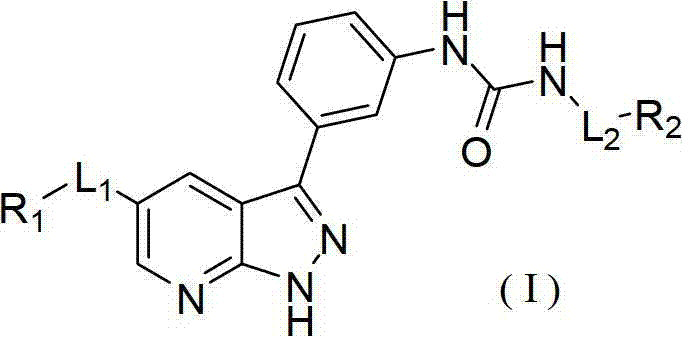
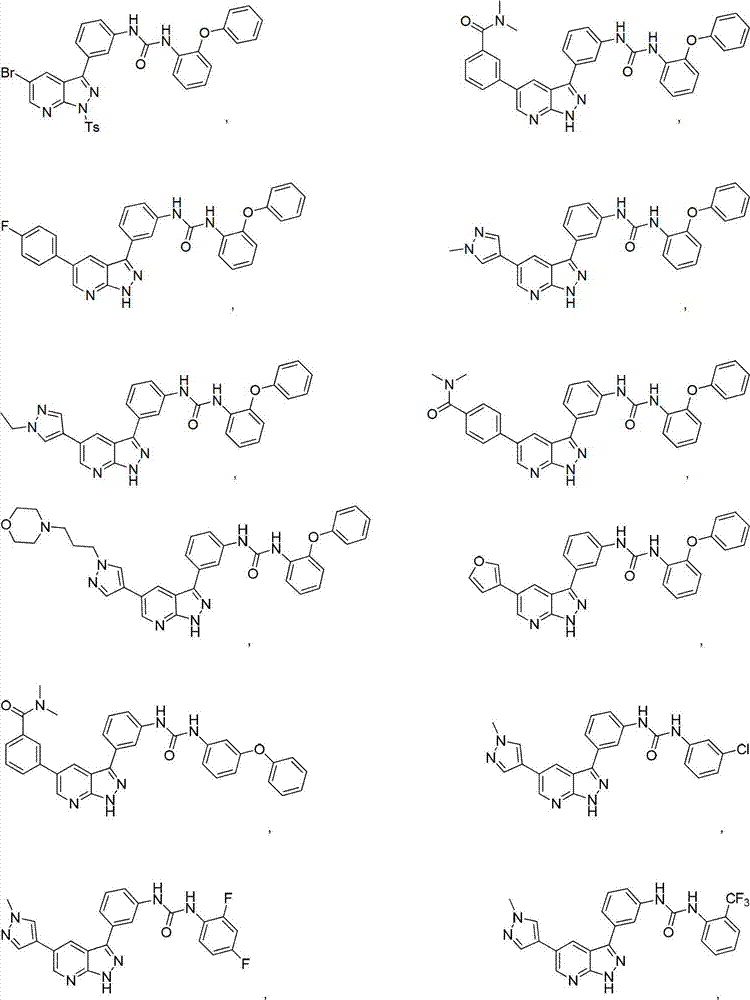
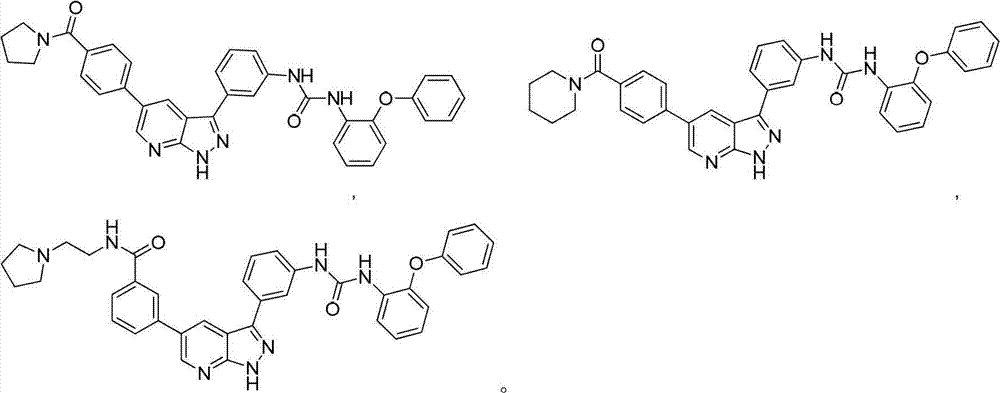
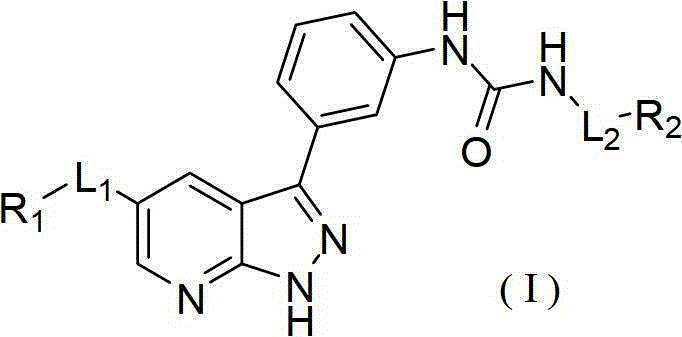
Pyrazolopyridine derivatives for inhibiting activity of insulin-like growth factor-1 receptor (IGF-1R) tyrosine kinase
The invention relates to pyrazolopyridine derivatives with the structure of formula (I) or pharmaceutically acceptable salts and application of the pyrazolopyridine derivatives or pharmaceutically acceptable salts. The invention also relates to a method for preparing compounds and an intermediate of the compounds.
Owner:JIANGSU SIMCERE PHARMA
Insulin-like growth factor 1 receptor-specific antibodies and uses thereof
ActiveUS10106614B2Efficient transportEasy to detectHybrid immunoglobulinsNervous disorderAntigen bindingBiology
Owner:NAT RES COUNCIL OF CANADA
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
Owner:OSI PHARMA INC
Combination therapies including inhibitors of the extracellular domain of E-cadherin
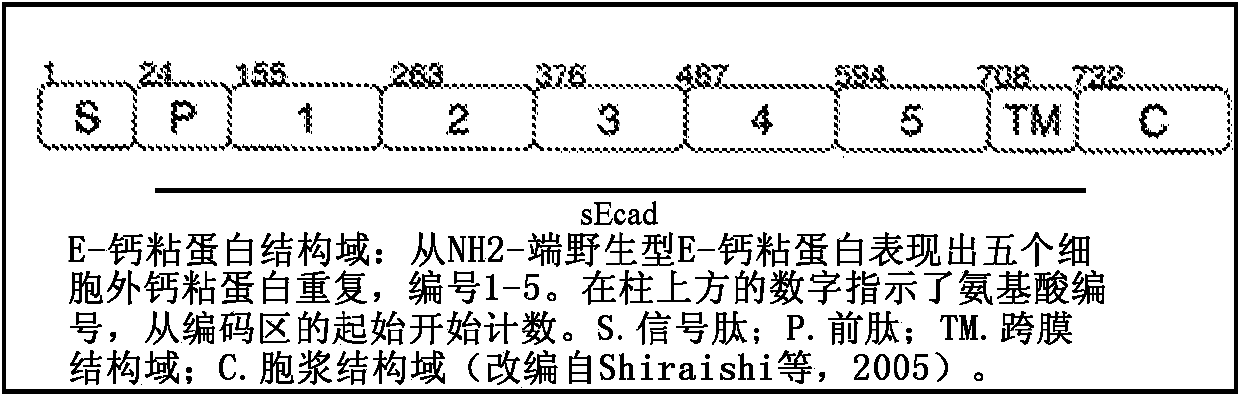
InactiveCN104519899AGrowth factor receptorPeptide/protein ingredientsGenetic material ingredientsTube formationLymphatic Spread
The present invention is based on our work with E-cadherin, including soluble portions of this integral membrane glycoprotein. The compositions of the present invention include therapeutically effective amounts of a first agent that targets epitopes within one or more of the EC2-EC5 subdomains of the ectodomain of E-cadherin (induing these domains in the shed sEcad fragment) and a second agent that inhibits one or more of: endothelial tube formation; angiogenesis; the human epidermal growth factor receptor family (i.e. HER1-4); an insulin-like growth factor 1 receptor (IGF-1R); any other receptor tyrosine kinase receptor family member; the P13K-MAPK pathway; and the P13K / Akt / mTOR pathway. The compositions can be used in the treatment of epithelial cancers, and may effectively inhibit cellular proliferation, migration, and / or invasiveness. Thus, the present compositions can be used to inhibit tumor growth and metastasis. The present compositions can be used in the treatment of "triple negative" breast cancer patients (in whom breast cancer cells test negative for estrogen receptors, progesterone receptors, and HER2), as well as HER2 -positive and other HER2 -negative tumors. In addition to therapeutic and prophylactic treatment methods, the invention features methods in which cancer is staged depending on the relative amounts of full-length (FL) E-cadherin and soluble E-cadherin (sEcad) and / or with another predictive biomarker for cancer. The greater the amount of sEcad relative to the amount of FL E-cadherin, the more advanced the cancer.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided.
Owner:OSI PHARMA INC
Combination therapy
ActiveUS8980259B2Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCarboxylic acidPyrrolidine
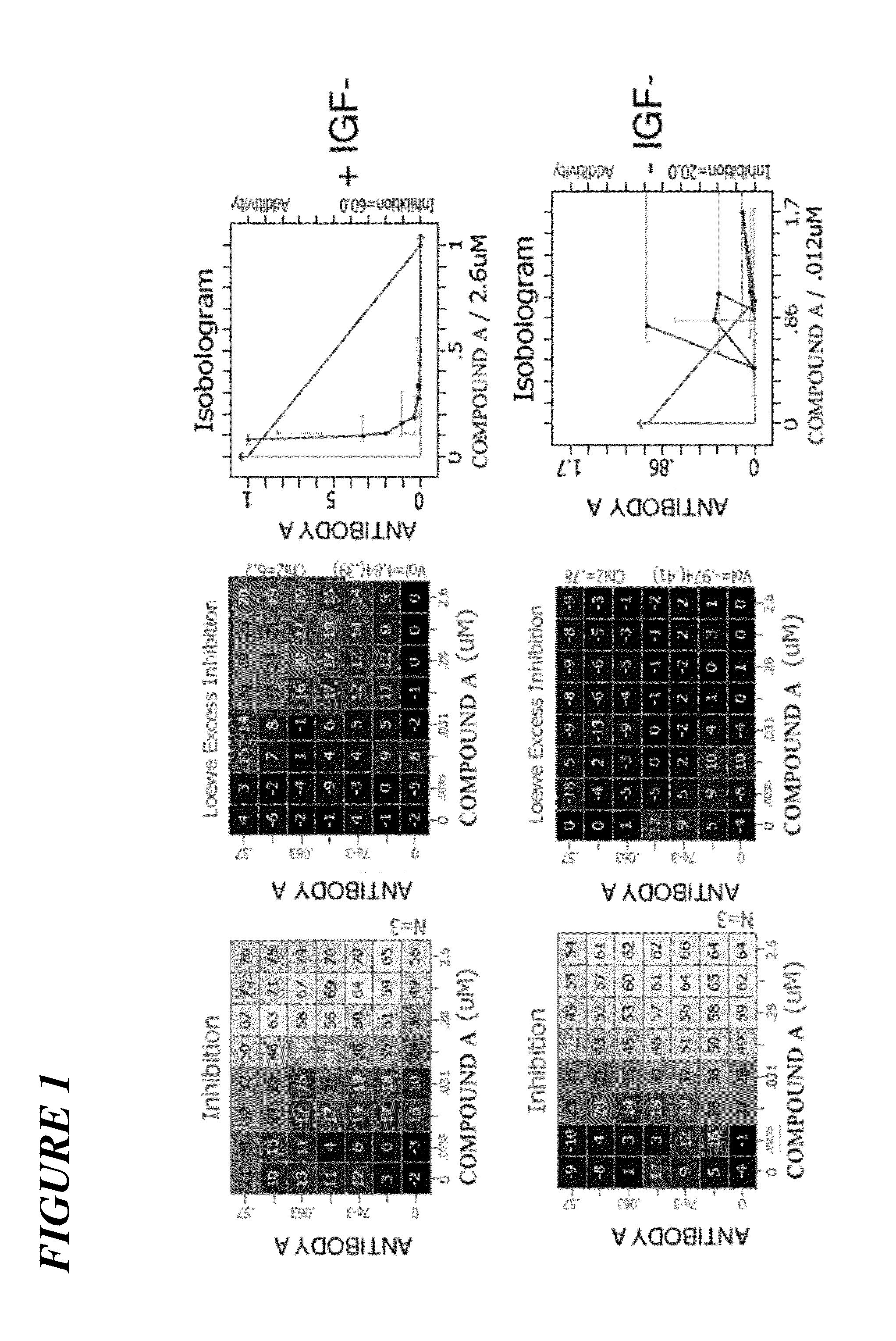
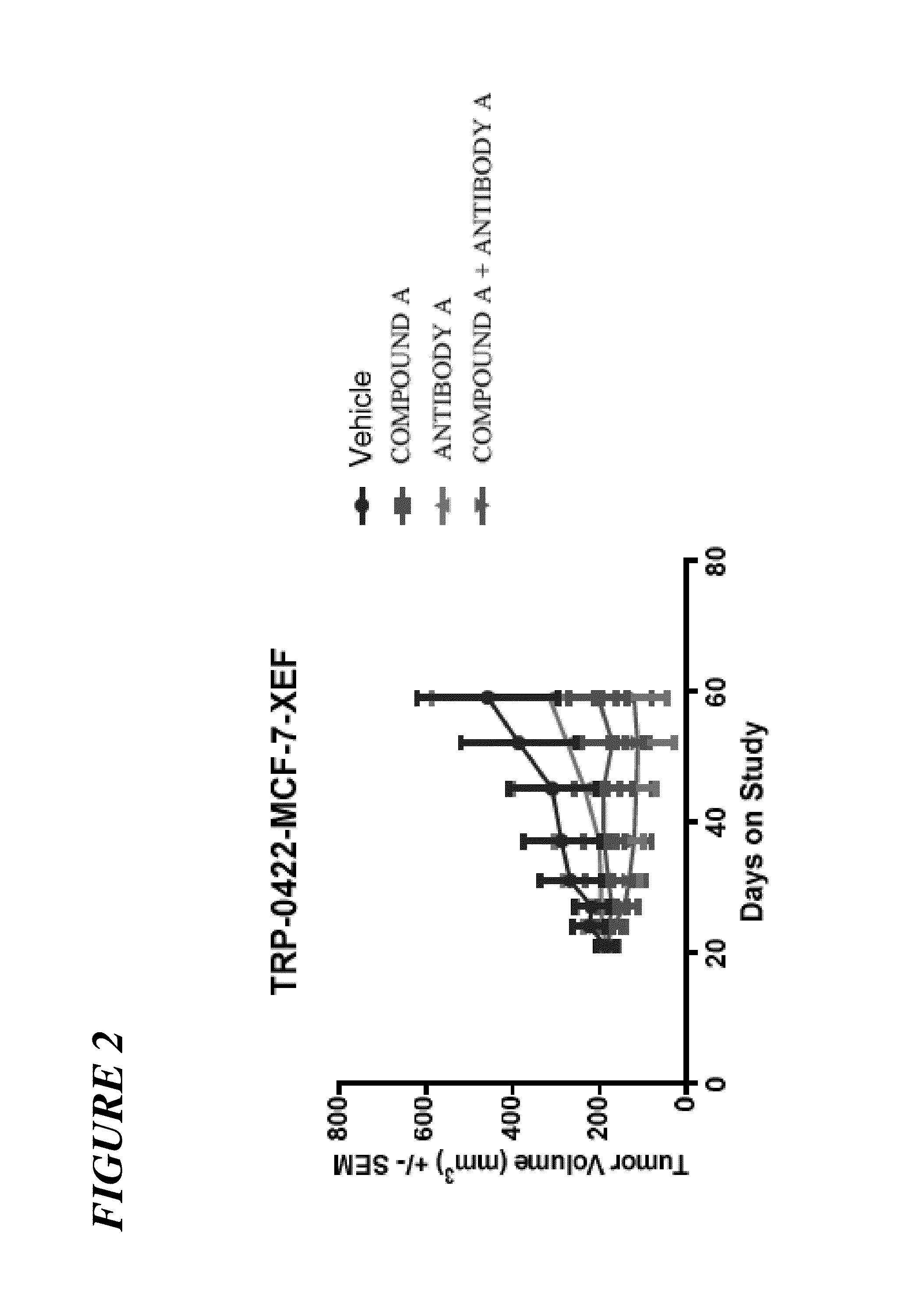
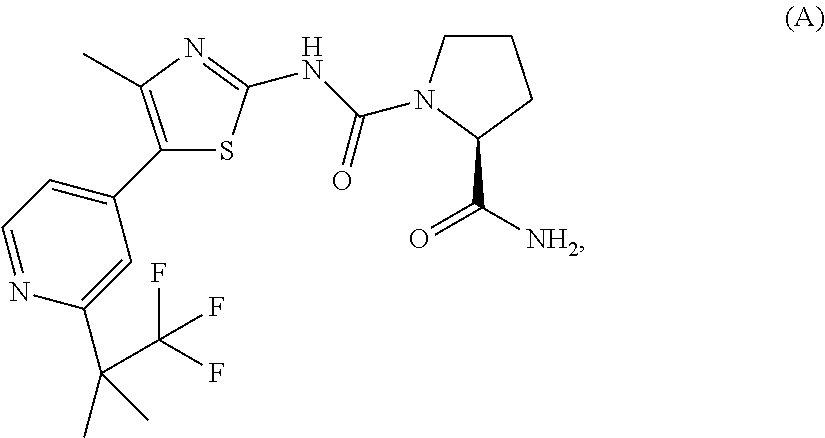
The present invention relates to a pharmaceutical combination comprising an alpha-isoform specific phosphatidylinositol 3-kinase inhibitor compound, such as (S)-Pyrrolidine-1,2-dicarboxylic acid 2-amide 1-({4-methyl-5-[2-(2,2,2-trifluoro-1,1-dimethyl-ethyl)-pyridin-4-yl]-thiazol-2-yl}-amide), or pharmaceutically acceptable salt thereof, and an insulin-like growth factor-1 receptor (IGF1R) inhibitor (e.g., the IGF1R inhibitor ANTIBODY A, or a variant or derivative thereof), a pharmaceutical composition comprising such combination, methods for treating cancer comprising administration of therapeutically effective amounts of such inhibitors to a subject in need thereof, and uses of such combination for the treatment of cancer.
Owner:AMGEN INC +1
Insulin-like growth factor 1 receptor-specific antibodies and uses thereof
ActiveUS10100117B2Efficient transportEasy to detectHybrid immunoglobulinsGeneral/multifunctional contrast agentsInsulin-like growth factorCereblon
The blood-brain barrier (BBB) prevents transport of molecules larger than 500 Dal tons from blood to brain. Receptor-mediated transcytosis (RMT) facilitates transport across the BBB of specific molecules that bind receptors on brain endothelial cells that form the BBB. An insulin-like growth factor 1 receptor (IGF 1R)-binding antibody or fragment thereof is identified that transmigrates the BBB by RMT. The antibody or fragment is used to deliver a cargo molecule across the BBB, wherein the cargo molecule may be a therapeutic or detectable agent. The antibody is a camelid VHH, prepared by immunizing a llama with a 933-amino acid IGF 1R polypeptide. Humanized forms of the camelid VHH are also generated.
Owner:NAT RES COUNCIL OF CANADA
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell expresses certain sensitivity or resistance biomarkers, or genomic classifiers. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate this methodology are also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
Pyrazolopyridine derivatives for inhibiting activity of insulin-like growth factor-1 receptor (IGF-1R) tyrosine kinase
Owner:JIANGSU SIMCERE PHARMA
Biological Markers Predictive Of Anti-Cancer Response To Insulin-like Growth Factor-1 Receptor Kinase Inhibitors
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided.
Owner:OSI PHARMA LLC
Insulin-like growth factor 1 receptor binding peptides
ActiveUS8415306B2Nervous disorderPeptide/protein ingredientsInsulin-like growth factorBinding peptide
The present invention relates to insulin-like growth factor 1 receptor binding peptides, polynucleotides encoding them, and methods of making and using the foregoing.
Owner:JANSSEN BIOTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com