Combination therapies including inhibitors of the extracellular domain of E-cadherin
A technology of extracellular domain and cadherin, which is applied in the field of combination therapy containing inhibitors of the extracellular domain of E-cadherin, which can solve the problem of low drug response rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
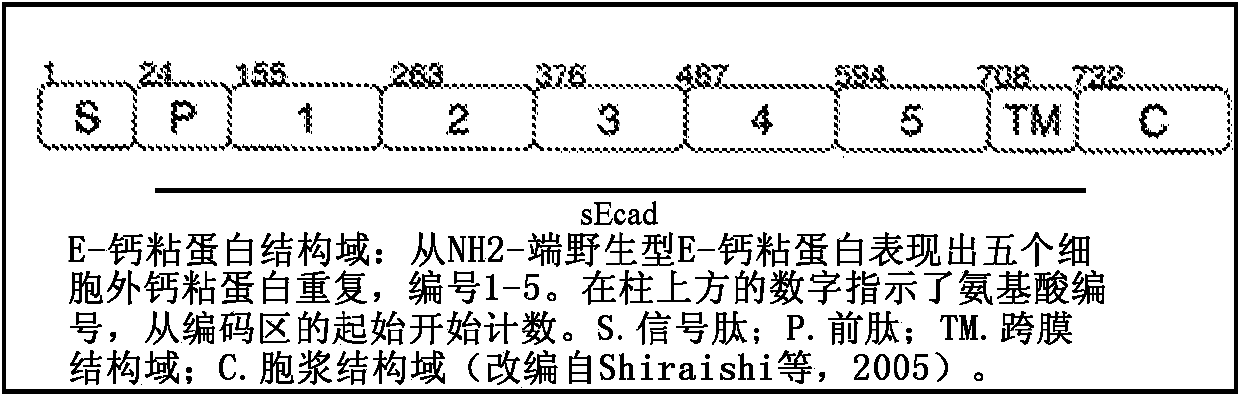
Image
Examples
Embodiment 1
[0102] Example 1: In vivo and in vitro efficacy of monoclonal antibodies targeting sEcad
[0103] We tested the in vivo efficacy of monoclonal antibodies targeting sEcad using MMTV-PyMT transgenic mice in which mammary gland-directed transgenic expression of the polyomavirus intermediate T antigen (PyMT) resulted in rapid degeneration of palpable tumors. occurs, the tumor develops into an aggressive adenocarcinoma that metastasizes to the lung (Guy et al., Mool. Cell Biol. 12 :954-961, 1992). Beginning at 47 days of age, mice were treated weekly with saline, IgG, or DECMA-1 (1 mg / kg in 200 μL of saline; Sigma) until sacrifice at 90 days of age. Treatment with anti-sEcad mAb caused a significant delay in tumor onset and reduced tumor burden (**p<0.01, ***p<0.001). Treated tumors exhibited reduced proliferation and increased apoptosis, the latter associated with increased p53 expression. Likewise, in HER2-positive (HER2+) Herceptin-resistant breast cancer xenografts, anti-sEc...
Embodiment 2
[0111] Example 2: Monoclonal antibodies against sEcad inhibit carcinogenesis by down-regulating multiple receptor tyrosine kinases and downstream MAPK-PI3K / Akt / mTOR and IAP pathways
[0112]Since the HER receptor family is an integral part of cancer growth and progression, we next assessed whether our anti-sEcad therapy inhibits tumorigenesis by modulating this pro-tumorigenic pathway. Our study revealed statistically significant expression levels of HER1, HER2, and IGF-1R in treated MMTV-PyMT (mainly HER1 and HER2) and cutaneous SCC xenograft tumors (mainly HER1, IGF-1R). significantly lower. As clinical trials combining HER-targeting agents with inhibitors of the PI3K / mTOR pathway are showing promise in patients (Hennessy et al., Nat. Rev. 4 :988-1004, 2005), we therefore next examined MAPK-PI3K / Akt / mTOR and IAP (i.e. survivin, livin, XIAP, c-IAP -1, C-IAP2, etc.) expression levels. Treated MMTV-PyMT tumors exhibited significant reductions in MEK1 / 2, ERK1 / 2, PI3K, Akt, mT...
Embodiment 3
[0124] Example 3: sEcad increases in human and mouse cancer and cell culture systems
[0125] Since sEcad has been previously reported to be upregulated in the serum of cancer patients (Katayama et al., Br J Cancer. 69 :580-585, 1994), we therefore first set out to determine whether endogenous sEcad levels were elevated in human or mouse cancer samples, body fluids or cell culture systems. To this end, we first assessed the expression levels of sEcad in HER2+ human breast tumor samples and human triple-negative breast cancer (TNBC) tumor samples and found significantly higher levels of sEcad compared with normal human breast tissue samples. In human cell culture studies, it was found that sEcad levels were significantly increased in conditioned medium of MCF-7 breast cancer cells compared to normal MCF-10A breast epithelial cells. In contrast, appreciable levels of sEcad were not found in HER2+SKBR3 and TNBCMDA-MB-231 cells, which could be attributed to the lack of E-cadherin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com