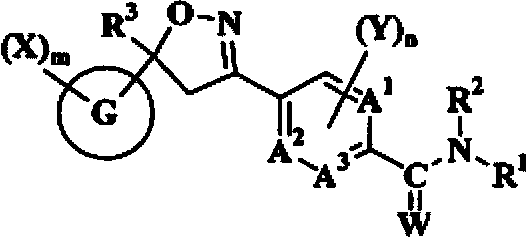
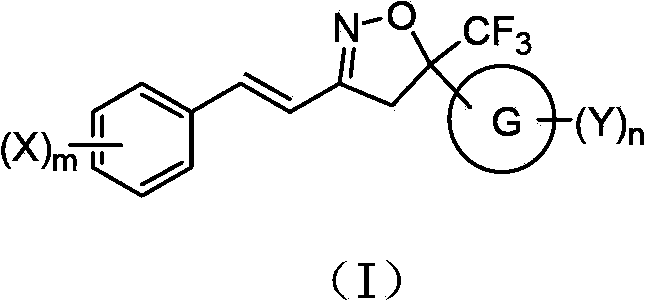
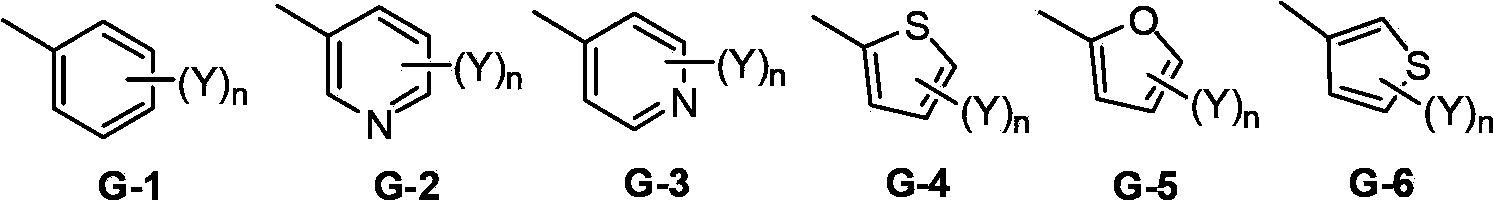A double-bond bridged trifluoromethylisoxazole compound, its preparation method and application
A technology of trifluoromethylisoxazole and compound, applied in the field of insecticide and bactericide, can solve the problem that the insecticidal and bactericidal activity of double bond bridge trifluoromethyl isoxazole compound has not been disclosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0083] The present invention will be further described below in conjunction with specific examples, but the present invention is not limited to these specific implementations. Those skilled in the art will realize that the present invention covers all alternatives, modifications and equivalents as may be included within the scope of the claims.
[0084] Synthesis Example 1
[0085] 3-[2-(3-Trifluoromethylphenyl)-vinyl]-5-trifluoromethyl-5-(3,4-dichlorophenyl)-4,5-dihydro-isoxazole Preparation of (compound 25)
[0086] Step 1: Synthesis of 3,3,3-trifluoro-2-(3,4-dichlorophenyl)propene
[0087]
[0088] Dissolve 10.2g of 3,4-dichlorophenylboronic acid in 80mL of tetrahydrofuran and 40mL of water, add 12.6g of 1,1,1-trifluoro-2-bromopropene, 17.4g of potassium carbonate and 0.84g of bis(triphenylphosphine) Palladium dichloride catalyst, reflux for 6 hours, add 50mL of water, filter, separate the filtrate, extract the water phase with ethyl acetate, dry over anhydrous sodium...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com