Method for preparing sulfachloropyrazine sodium solution serving as compound anti-coccidium medicine
A technology of sulfachlorpyrazine sodium and drug solution, which is used in drug delivery, drug combination, anti-infective drugs and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
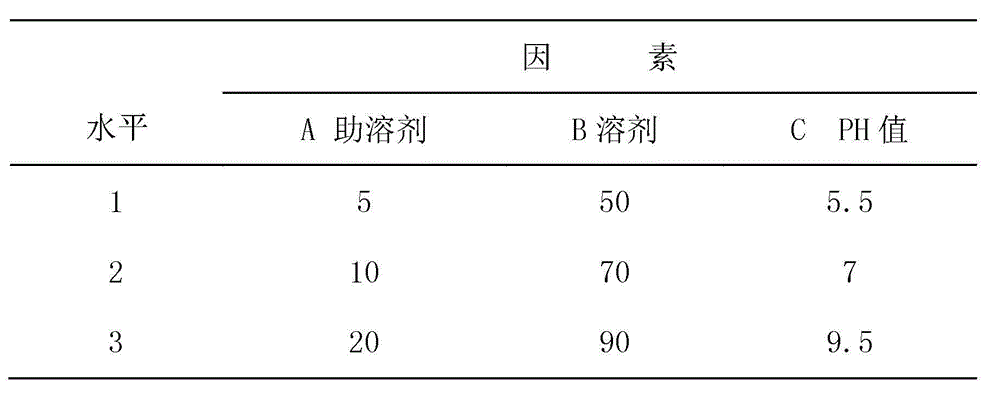
[0053] Accurately weigh 30kg of dimethyl sulfoxide, 40kg of N-methylpyrrolidone and 10kg of sulfachlorpyrazine sodium and pour them into the liquid mixing tank at room temperature and stir until dissolved. Mix 2kg of ditrimethoprim with 10kg of glacial acetic acid and dissolve at low temperature , pour the above solution, and finally adjust the pH value (7) with triethanolamine, and set the volume to 100kg with N-methylpyrrolidone. The medicinal solution is then filtered through a 0.45 μm microporous filter element, and then sterilized at 100° C. for 30 minutes to obtain the product.
Embodiment 2
[0055] Accurately weigh 35kg of dimethyl sulfoxide, 30kg of N-methylpyrrolidone and 15kg of sulfachlorpyrazine sodium, pour them into the liquid mixing tank and stir at room temperature until dissolved, mix 3kg of ditrimethoprim with 5kg of lactic acid and dissolve at a low temperature. Pour the above solution, and finally adjust the pH value (6.5) with triethanolamine, and set the volume to 100kg with N-methylpyrrolidone. The medicinal solution is then filtered through a 0.45 μm microporous filter element, and then sterilized at 100° C. for 30 minutes to obtain the product.
Embodiment 3
[0057] Accurately weigh 30 kg of dimethyl sulfoxide, 50 kg of N-methylpyrrolidone and 5 kg of sulfachlorpyrazine sodium and pour them into the liquid mixing tank and stir at room temperature until dissolved, then add 2 kg of trimethoprim dissolved in 15 kg of glacial acetic acid, Finally, adjust the pH value (8.0) with triethanolamine, and set the volume to 100kg with N-methylpyrrolidone. The medicinal solution is then filtered through a 0.45 μm microporous filter element, and then sterilized at 100° C. for 30 minutes to obtain the product.
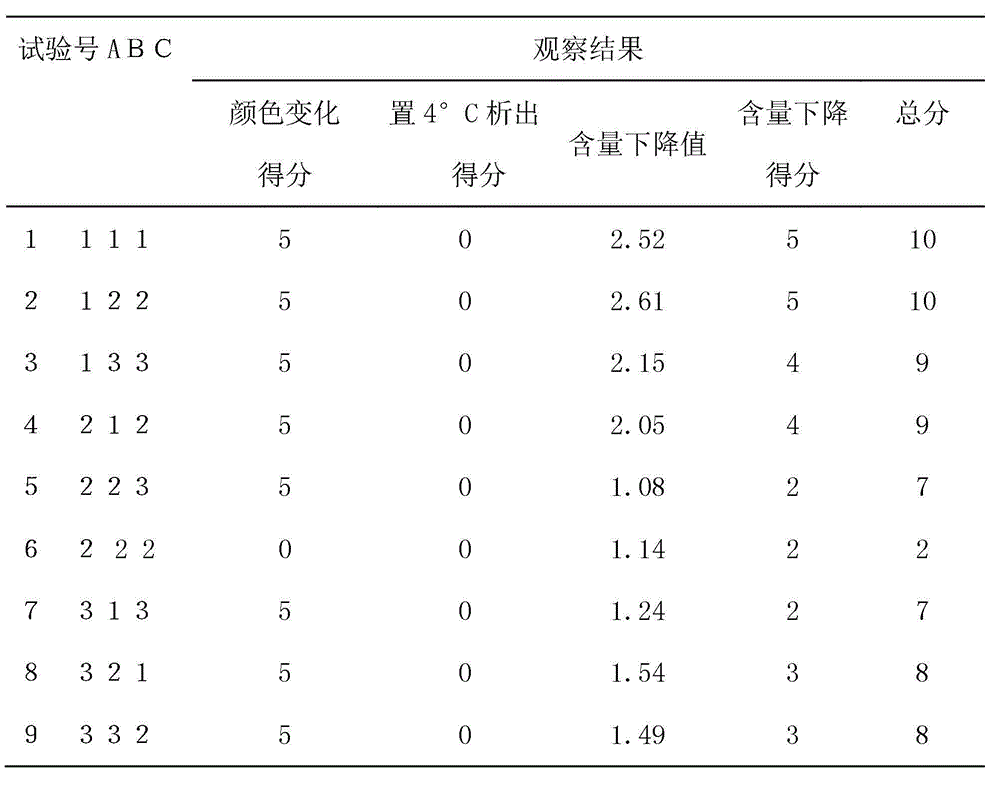
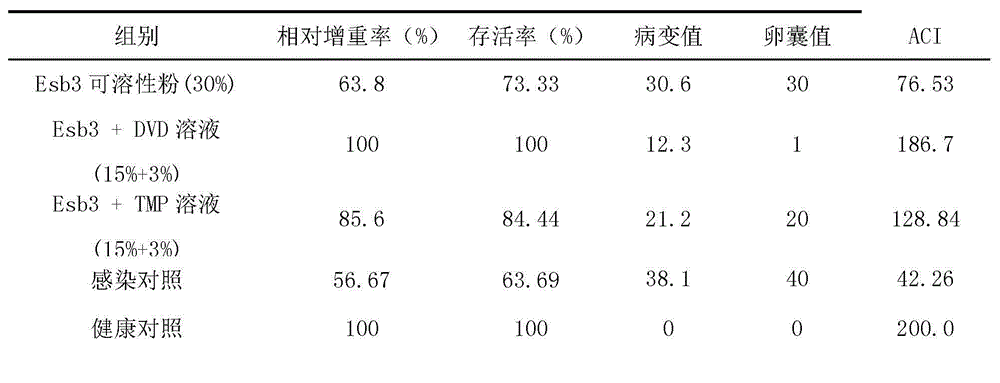
[0058] The invention has the advantages of simple production process, stable product quality, and can be placed at room temperature for two years without deterioration. The medicine is a solution, which is more convenient to administer and divide doses than the existing dispersant, and is especially suitable for large-scale breeding plants, and can greatly reduce the labor intensity of people when administering medicine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com