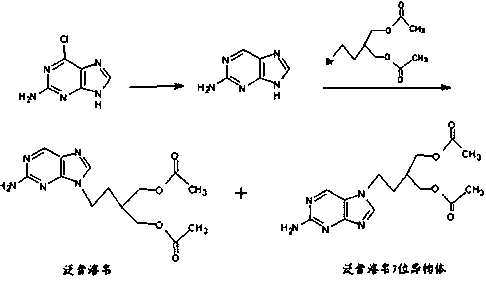
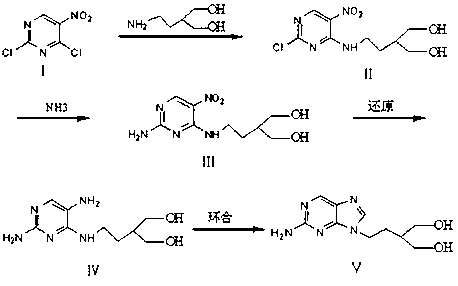
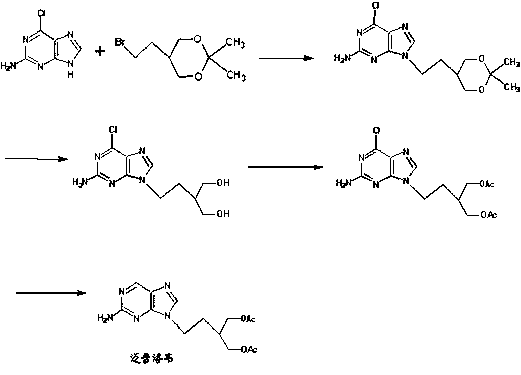
Synthetic method of famciclovir intermediate
A technology of famciclovir intermediate and synthetic method, which is applied in the field of synthesis of famciclovir intermediate, can solve the problems of low total yield, long reaction steps, and reduced product purity, and achieve the effects of high yield, few steps, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve 50g (0.259mol) of 2,4-dichloro-5-nitropyrimidine in 600ml of ethanol, stir at 20-30°C, then add 46.5g (0.390mol) of 2-(2-aminoethyl)propanediol and 41g of sodium carbonate , reacted at 50-60°C for 12 hours, filtered while it was hot, cooled the filtrate to 0-10°C, and gradually precipitated light yellow crystals, filtered to obtain 2-{2-[(2-chloro-5-nitropyrimidin-4-yl ) amino] ethyl}-1,3-propanediol 56g, yield 78%.
Embodiment 2
[0034] Dissolve 30g (0.109mol) of 2-{2-[(2-chloro-5-nitropyrimidin-4-yl)amino]ethyl}-1,3-propanediol in 300ml of methanol, stir at 30-40°C, and pass through Add ammonia gas for ammonolysis for 15 hours, cool at 0-10°C, stir for 4 hours, filter the precipitated light yellow crystals to obtain 2-{2-[(2-amino-5-nitropyrimidin-4-yl)amino]ethyl Base}-1,3-propanediol 24g, yield 86%.
Embodiment 3
[0036] Mix 40g (0.156mol) of 2-{2-[(2-amino-5-nitropyrimidin-4-yl)amino]ethyl}-1,3-propanediol with 600ml of ethanol, stir and dissolve at 30-40℃, then Add 5 g of 10% palladium carbon, hydrogenate under normal pressure for 10 hours, filter, and concentrate the filtrate to remove the solvent to obtain 2-{2-[(2-amino-5-aminopyrimidin-4-yl)amino]ethyl}-1 , 35g (theoretical amount) of 3-propanediol does not need to be purified and can be directly used in the cyclization reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com