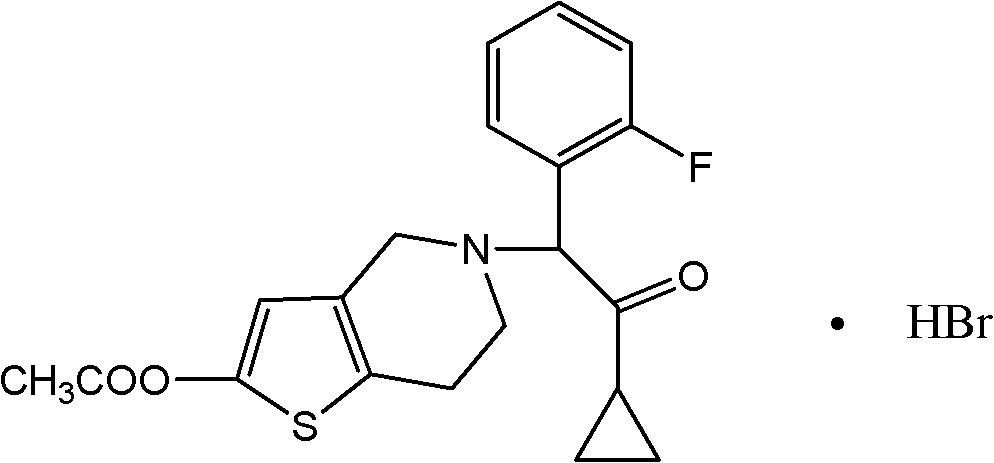
Prasugrel hydrobromide tablet
A technology of hydrobromic acid and tablets, which is applied in the field of prasugrel hydrobromide tablets, can solve the problems of accelerating the hydrolysis and oxidation of prasugrel, the hazards of antioxidants and colorants, and the difficulty of production and operation, and achieve Good bioavailability, good stability, good effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Preparation method: Pass prasugrel hydrobromide through a 120-mesh sieve, take the prescribed amount of prasugrel hydrobromide that has been sieved and mix with the prescribed amount of starch-lactose compound in equal increments, add the prescribed amount of magnesium stearate and mix evenly , measure the content of the semi-finished product, calculate the weight of the tablet, and press the tablet.
Embodiment 2
[0033]
[0034] The preparation method is the same as in Example 1.
Embodiment 3
[0036]
[0037] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com