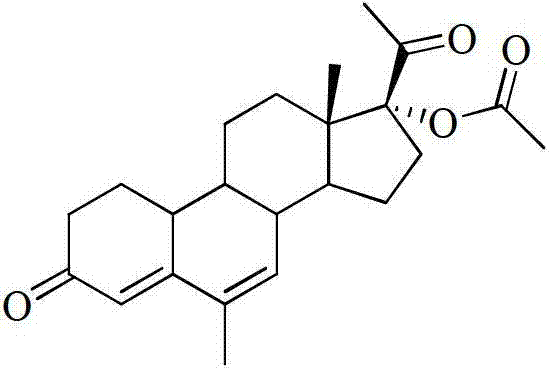
Synthetic method of 6-methyl-17alpha-acetoxyl-19-norpregna-4,6-dialkyl-3,20-diketone
A technology of acetoxy and methylpregnant, applied in the field of compound synthesis, can solve problems such as difficulty in obtaining, and achieve the effects of simplified operation, simple reaction operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
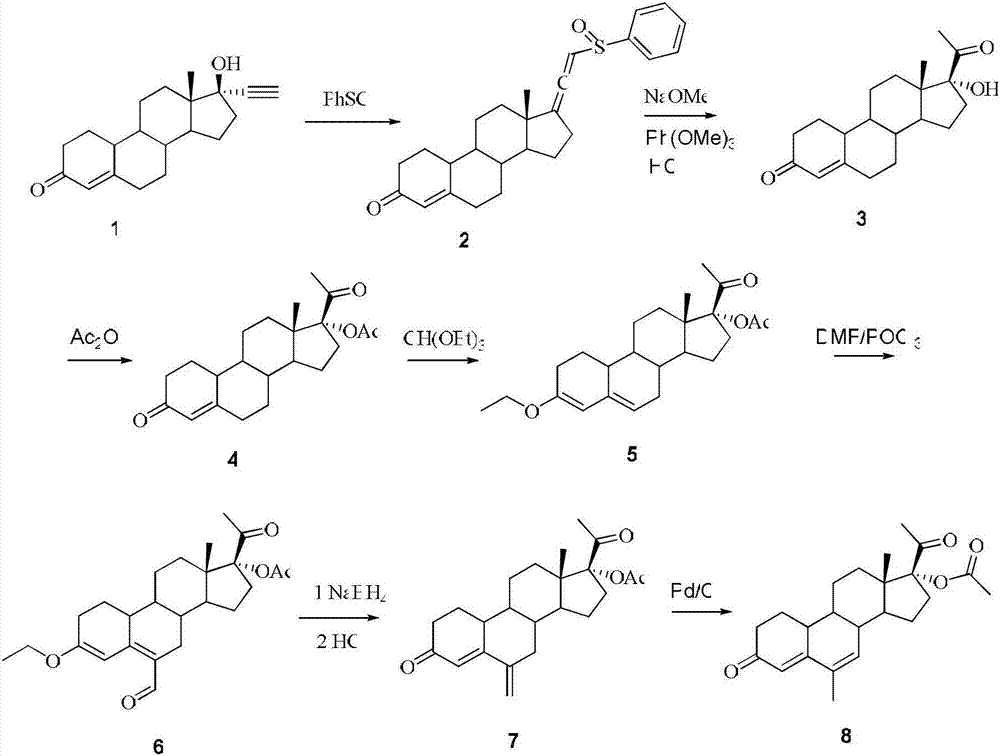
Embodiment 1
[0027] 1.1, 19-desmethyl-21-phenylsulfinylpregna-4,17(20),20-triene (2)
[0028] Add compound 1 (17α-ethynyl-17β-hydroxy-19-normethylpregn-4-en-3-one) (10 g, 33.5 mmol), dichloromethane (500 ml), triethylamine into the reaction flask (17g, 167.5mmol), cooled in an ice bath below -10°C, added dropwise a dichloromethane solution (25ml) of benzenesulfenyl chloride (9.68g, 67mmol), reacted at -10°C for 20 minutes, added water (150ml) and Methanol (30ml), stirred and separated, washed with dilute hydrochloric acid and saturated NaCl solution, dried, concentrated the solvent, recrystallized to obtain a white solid product, dried and weighed 11.4g, yield 85%. HNMR (ppm, CDCl 3 ): 7.63-7.66(m,2H),7.46-7.55(m,3H),6.11-6.15(m,1H),5.83(s,1H),2.65-2.71(m,1H),2.47-2.58(m ,2H),2.38-2.44(dd,1H),2.19-2.30(m,3H),2.08-2.13(m,1H),1.82-1.89(m,4H),1.08-1.65(m,8H),0.95 (s,3H).
[0029] 1.2, 17α-hydroxy-19-desmethylpregn-4-ene-3,20-dione (3)
Embodiment 2
[0042] 2.1, 19-desmethyl-21-phenylsulfinylpregna-4,17(20),20-triene (2)
[0043] Add compound 1 (10g, 33.5mmol), dichloromethane (500ml), triethylamine (17g, 167.5mmol) into the reaction flask, cool to -5°C, dropwise add benzenesulfenyl chloride (9.68g, 67mmol) Dichloromethane solution (35ml), keep the reaction temperature at -5°C for 30 minutes, add 150ml of water and 30ml of methanol, stir for 10 minutes, separate layers, wash with saturated NaCl solution, dry, concentrate the solvent, recrystallize to obtain a white solid product, The dried weight is 12.3g, and the yield is 90% (the product purity is the same as in Example 1.1).
[0044] 2.2, 17α-hydroxy-19-desmethylpregna-4-ene-3,20-dione (3)
[0045] Add methanol (500ml) and metal sodium (1.13g, 47.2mmol) into the reaction flask, add compound 2 (24g, 59mmol) after the reaction, raise the temperature to 50°C for 2 hours, add trimethyl phosphite (8.12g, 64.9 mmol), reacted for 1 h, added HCl, poured into water, precipitat...
Embodiment 3
[0057] 3.1, 19-desmethyl-21-phenylsulfinylpregna-4,17(20),20-triene (2)
[0058] Add compound 1 (10g, 33.5mmol), dichloromethane (500ml), triethylamine (17g, 167.5mmol) into the reaction flask, cool in an ice bath at 0°C, dropwise add benzenesulfenyl chloride (9.68g, 67mmol) Dichloromethane solution (35ml), keep the reaction temperature at -0°C for 30 minutes, add 150ml of water and 30ml of methanol, stir for 10 minutes, separate layers, wash with saturated NaCl solution, dry, concentrate the solvent, and recrystallize to obtain a white solid product, The dried weight is 11g, and the yield is 80% (the product purity is the same as in Example 1.1).
[0059] 3.2, 17α-hydroxy-19-desmethylpregn-4-ene-3,20-dione (3)
[0060]Add methanol (500ml) and metal sodium (1.13g, 47.2mmol) to the reaction flask, add compound 2 (24g, 59mmol) after the reaction, raise the temperature to 65°C for 2 hours, add trimethyl phosphite (8.12g, 64.9 mmol), reacted for 1 hour, cooled, added HCl, poured...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com