Modification method of lithium-rich cathode material
A technology of lithium-rich positive electrode materials and positive electrode materials, applied in battery electrodes, electrical components, circuits, etc., can solve the problems of reducing the discharge capacity of materials, achieve low cost, increase rate capacity, and reduce the effect of irreversible capacity loss for the first time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. According to the molecular formula Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 The ratio of Li, Ni, Mn to prepare LiAc, Ni(Ac) 2 and Mn(Ac) 2 Mixed solution, the total metal (Li+Ni+Mn) ion concentration is 1mol / L;
[0022] 2. Configure the citric acid solution according to the ratio of citric acid: total metal ions = 1:1 (molar ratio). Under stirring conditions, add the mixed solution of metal salts to the citric acid solution dropwise, stir and evaporate in a water bath at 80°C, and finally A yellow-green gel was obtained, and after drying in a vacuum oven at 150°C for 12 hours, a dry and slightly fluffy bulk precursor was obtained; since the aforementioned citric acid solution was prepared by adding deionized water, in the subsequent evaporation process The deionized water is completely removed, so the deionized water has no other effects except for dissolving citric acid, so the added deionized water can completely dissolve the citric acid or a little more. The amount of de...
Embodiment 2
[0029] 1-3 steps are the same as embodiment 1;
[0030] 4. Disperse 0.99 g of the lithium-rich cathode material prepared above in 100 ml of FeC with a concentration of 0.095 g / L 2 o 4 Sonicate the solution for 1 hour, then vigorously stir for 2 hours, and drop 50ml of NH with a concentration of 0.076g / L into it 4 h 2 PO 4 solution, stirred for 1 h to make it evenly mixed, and the resulting slurry was completely dried at 85°C.
[0031] 5. Then sinter at 400°C for 6 hours to obtain surface-modified Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 .
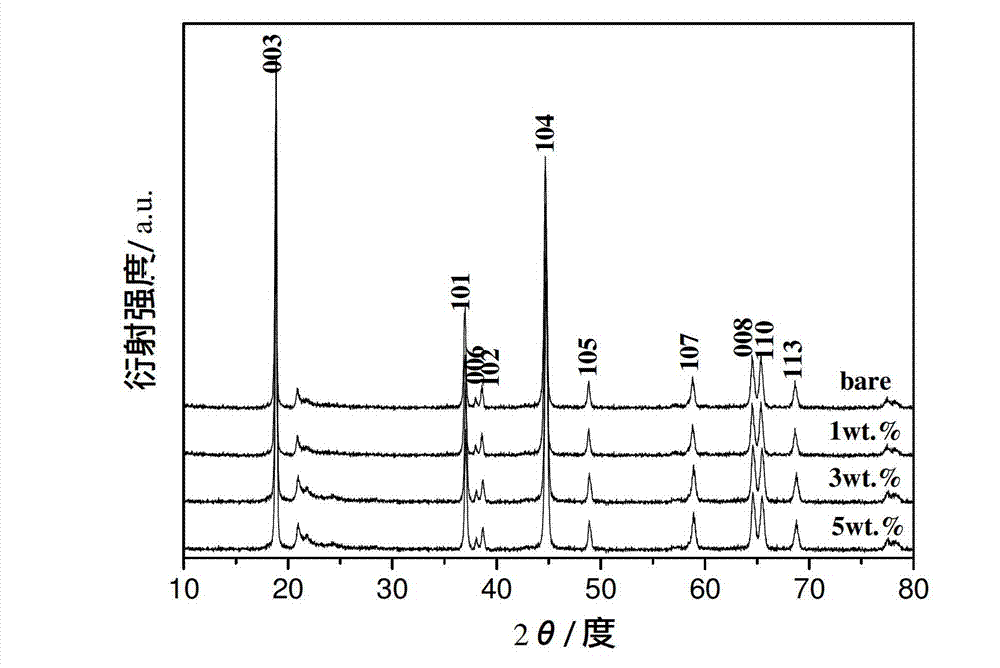
[0032] X-ray diffraction (XRD) analysis showed that the main phase of the product was Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 ( figure 1 The map corresponding to 1wt.% in ), the structure has not been destroyed after coating.
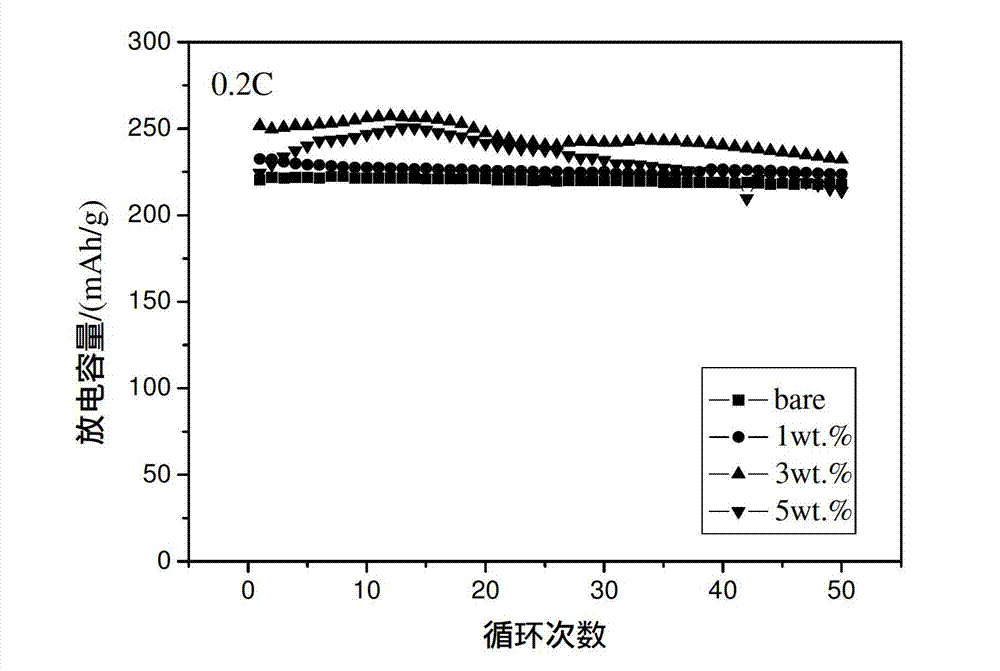
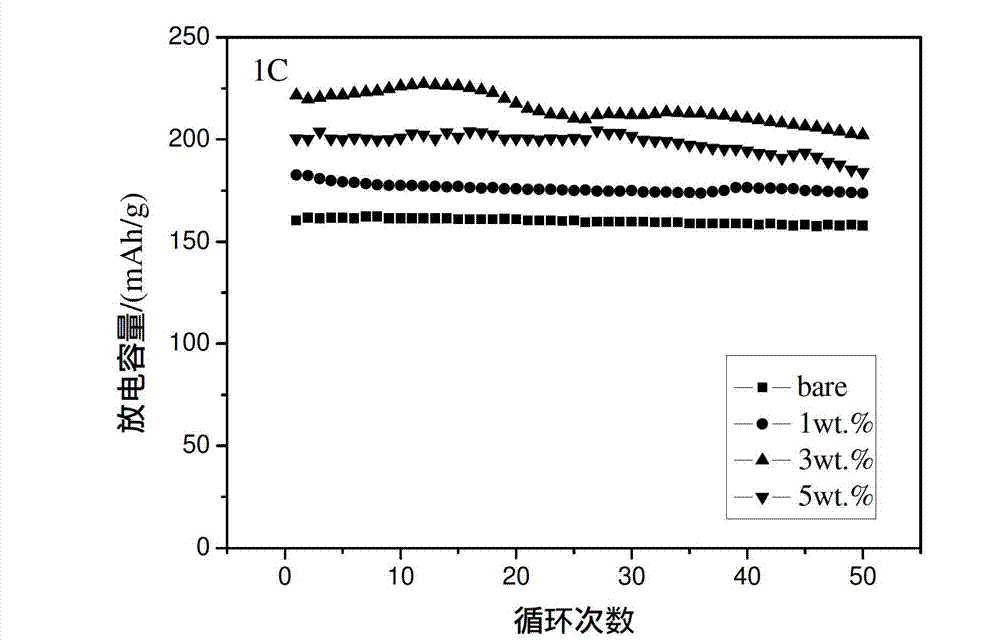
[0033] Electrochemical tests show that when charging and discharging at 1C and 2C, the initial discharge capacity is 150mAh / g (see image 3 ) and 138mAh / g (see Figure 4 ), after 50 cycles, it still maintains a high capacit...
Embodiment 3
[0035] 1-3 steps are the same as embodiment 1;
[0036] 4. Disperse 0.95 g of the obtained lithium-rich cathode material in 100 ml of FeC with a concentration of 0.47 g / L 2 o 4 Sonicate the solution for 1h, then stir vigorously for 2h, and drop 50ml of NH with a concentration of 0.38g / L into it 4 h 2 PO 4 solution, stirred for 1 h to make it evenly mixed, and the resulting slurry was completely dried at 85°C.
[0037] 5. Then sinter at 400°C for 6 hours to obtain surface-modified Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 .
[0038] X-ray diffraction (XRD) analysis showed that the main phase of the product was Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 ( figure 1 The map corresponding to 5wt.% in the middle), and its structure has not been destroyed after coating.
[0039] Electrochemical tests show that when charging and discharging at 1C and 2C, the first discharge capacity is 200mAh / g (see image 3 ) and 180mAh / g (see Figure 4 ), after 50 cycles, it still maintains a high capacity, in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com