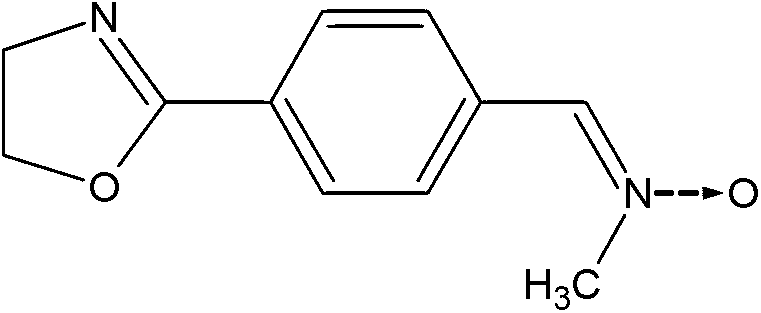
Graft polymer to which combined nitrogen molecules are grafted
A technology for polymers and compounds, applied in organic chemistry and other directions, can solve problems such as reducing hysteresis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1: By grafting 2,4,6-trimethyl-3-(2-(2-oxyimidazolidin-1-yl)ethoxy) Nitrile Oxide Modified SBR
[0106] 1.1- Preparation of Modifier:
[0107] a) Preparation of 1-(2-(3'-nitrileoxytrimethylphenyl-1'-oxyl)ethyl)imidazolidin-2-one
[0108]
[0109] This compound can be prepared from hydroxyethylimidazolidinone tricresylphenol according to the following synthetic scheme.
[0110]
[0111] b) Preparation of 3-hydroxy-2,4,6-trimethylbenzaldehyde
[0112]
[0113] This compound can be obtained according to the procedures described in the following articles: Yakubov, A.P.; Tsyganov, D.V.; Belen'kii, L.I.; Krayushkin, M.M.; Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science (English Translation) ; vol.40; nb.7.2; (1991); pp. 1427-1432; Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya; nb.7;
[0114] c) Preparation of 1-(2-chloroethyl)imidazolidin-2-one:
[0115]
[0116] This product is described in the article Naga...
Embodiment 2
[0174] Example 2: By grafting 2,4,6-trimethyl-3-(2-(2-oxoimidazolidin-1-yl)ethoxy) Bulk Modified Polyisoprene
[0175] 2.1- Modifier grafted to polyisoprene Natsyn2200 (Goodyear)
[0176] The same modifier as that obtained in Example 1 before was used.
[0177] 2.85 g of 2,4,6-trimethyl-3-(2-(2-oxoimidazolidin-1-yl ) ethoxy) nitrile oxide doped with 30 g of polyisoprene Natsyn 2200 ([ML(1+4) 100°C=79, unit 3,4=0.5%, unit trans 1,4=1.9%, unit cis 1,4=97.6%, Mw=1044.10 3 g / mol, Vp=3.6]). The mixture was homogenized 15 times.
[0178] This mixing stage was followed by a heat treatment in a press at a pressure of 10 bar.
[0179] The duration and temperature of this second phase are adjusted.
[0180] 1 H NMR analysis made it possible to determine the molar amount of grafting and the molar yield of grafting, which are reported in the table below:
[0181] Table 6
[0182]
[0183]
Embodiment 3
[0184] Example 3: By grafting 3-methoxy-4-[2-(2-oxyimidazolidin-1-yl)ethoxy]benzyl Nitrile Oxide Modified SBR
[0185] 1.1- Preparation of Modifier:
[0186] a) Preparation of 3-methoxy-4-[2-(2-oxyimidazolidin-1-yl)ethoxy]benzonitrile oxide
[0187]
[0188] This compound can be prepared from vanillin and 2-chloroethyl imidazolidinone according to the following synthetic scheme:
[0189]
[0190] b) Preparation of 3-methoxy-4-[2-(2-oxyimidazolidin-1-yl)ethoxy]benzaldehyde
[0191]
[0192] Route A
[0193] Vanillin (30.0g, 0.197mol) and K 2 CO 3 (95.4 g, 0.690 mol) in DMF (200 mL) was heated at 50°С for 15 min. To this suspension was added 1-(2-chloroethyl)imidazolidinone-2-one (44.0 g, 0.296 mol, purity >90%) in DMF (30 mL), 1-(2-chloroethyl The preparation of the imidazolidinone-2-one has been described in Example 1. The reaction medium is heated to 90°C (T 浴 ), the temperature is maintained for about 4 hours. The reaction medium was allowed to come...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com