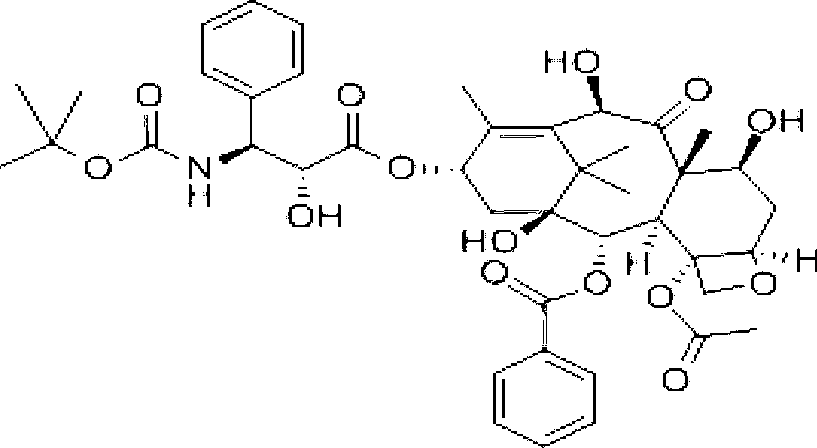
Capecitabine dispersible tablet and preparation method thereof
A technology of capecitabine and dispersible tablets, which is applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, and pharmaceutical formulas, and can solve the problems that the stability and dissolution rate are difficult to meet the requirements of the Pharmacopoeia. Achieve good application prospects, enhance bioavailability, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
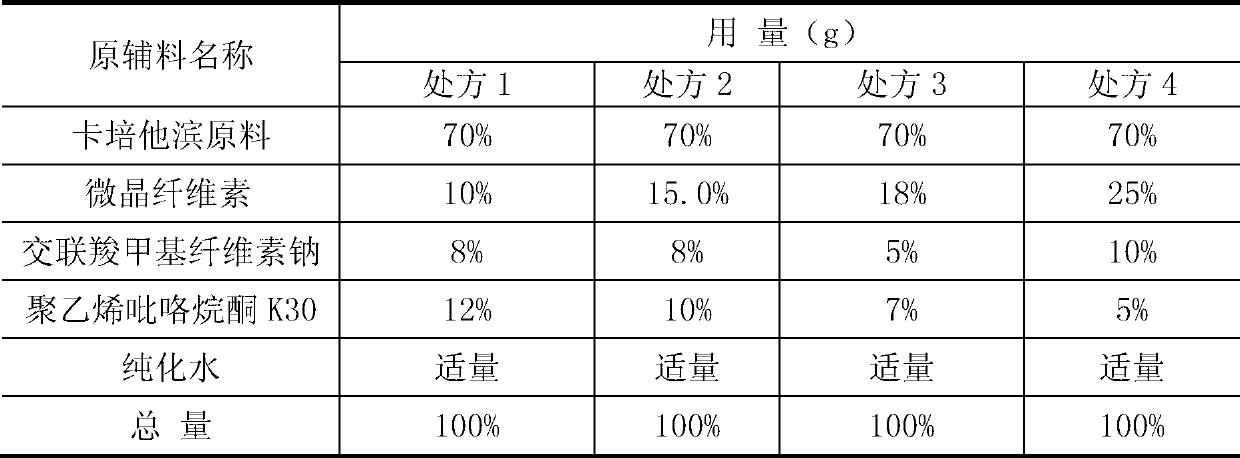
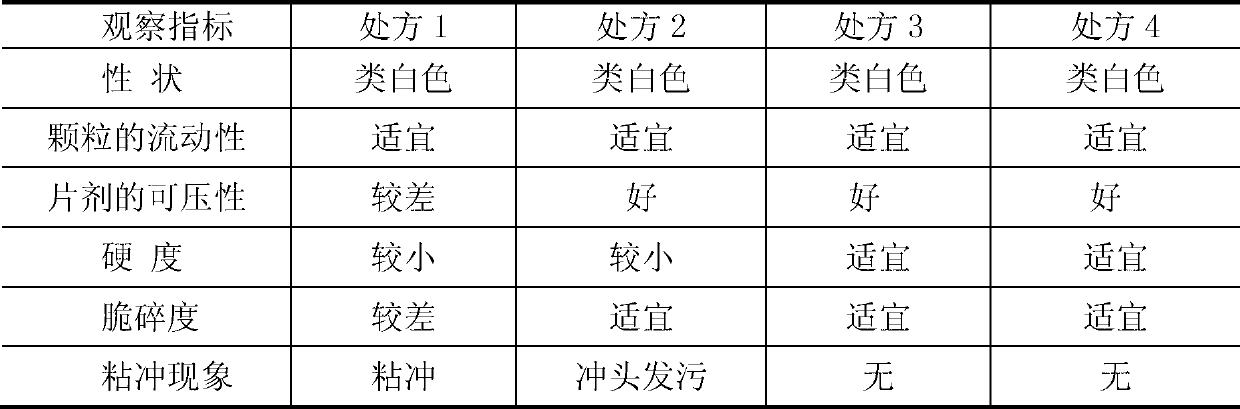
[0066] Add 75% capecitabine to a 120-mesh sieve, microcrystalline cellulose 10% (w / w), and croscarmellose sodium 15% (w / w) into a three-dimensional motion mixer and mix thoroughly Uniformly; put the uniformly mixed material into a high-efficiency wet granulator, and set aside; add 5% polyvinylpyrrolidone K30 soft material, pass through a 20-mesh sieve, dry at 50°C, dry for 6 hours, granulate, and mix Uniform, ready to be pressed into tablets.
Embodiment 2
[0068] Add 80% capecitabine to a 120-mesh sieve, microcrystalline cellulose 7.5% (w / w), and croscarmellose sodium 7.5% (w / w) into a three-dimensional motion mixer and mix thoroughly Uniformly; put the uniformly mixed material into a high-efficiency wet granulator, and set aside; add 5% polyvinylpyrrolidone K30 soft material, pass through a 20-mesh sieve, dry at 50°C, dry for 6 hours, granulate, and mix Uniform, ready to be pressed into tablets.
Embodiment 3
[0070] Add capecitabine 75%, microcrystalline cellulose 15% (w / w), and croscarmellose sodium 7.5% (w / w) into a three-dimensional motion mixer, and mix well; Put the material into a high-efficiency wet granulator, and set it aside; add 2.5% polyvinylpyrrolidone K30 soft material, pass through a 20-mesh sieve, dry, granulate, mix evenly, and tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mesh | aaaaa | aaaaa |
| Mesh | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com