Method for enhancing secretion of glucose oxidase by coexpression of UPR (unfolded protein response) key genes and downstream target genes
A glucose oxidase and key gene technology, applied in the direction of microorganism-based methods, oxidoreductase, biochemical equipment and methods, etc., can solve the problems of high cost, complicated separation and extraction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Construction and Identification of Embodiment 1 Recombinant Bacteria
[0038] 1) Design primers to amplify Haclp, Pdi1, Ero1, Lhs1, Kar2, Sil1 genes using Pichia pastoris and Saccharomyces cerevisiae reverse transcription cDNA as template;
[0039] Hac-F: 5'-A G TTCGAA ATGCCCGTAGATTCTTCTC-3'
[0040] Hac-R: 5'-AAATAT GCGGCCGC CTATTCCTGGAAGAATACAAAGTC-3'
[0041] Pdi-F: 5'-CG GGATCC ATGCAATTCAACTGGGATATTAAAACTGTG-3'
[0042] Pdi-R: 5'-AAATAT GCGGCCGC TTAAAGCTCGTCGTGAGCGTC-3'
[0043] Ero-F: 5'-CG GGATCC ATGAGATTAAGAACCGCCAT-3'
[0044] Ero-R: 5'-AAATAT GCGGCCGC TTATTGTATATCTAGCTTAT-3'
[0045] Lhs-F: 5'-CG GGATCC ATGCGAAACGTTTTAAGGCTTTT-3'
[0046] Lhs-R: 5'-AAATAT GCGGCCGC CTATAATTCATCATGCAAAATGTCT-3'
[0047] Kar-F: 5'-CG GGATCC ATGCTGTCGTTAAAACCATCTT-3'
[0048] Kar-R: 5'-AAATAT GCGGCCGC TATCTACAACTCATCATGAT-3'
[0049] Sil-F: 5'-CG GGATCC ATGGTCCGGATTCTTCCCAT-3'
[0050] Sil-R: 5'-AAATAT GCGGCCGC TCAGAGTTCATCTCTGAAATTT-3'
[0051] Add t...
Embodiment 2
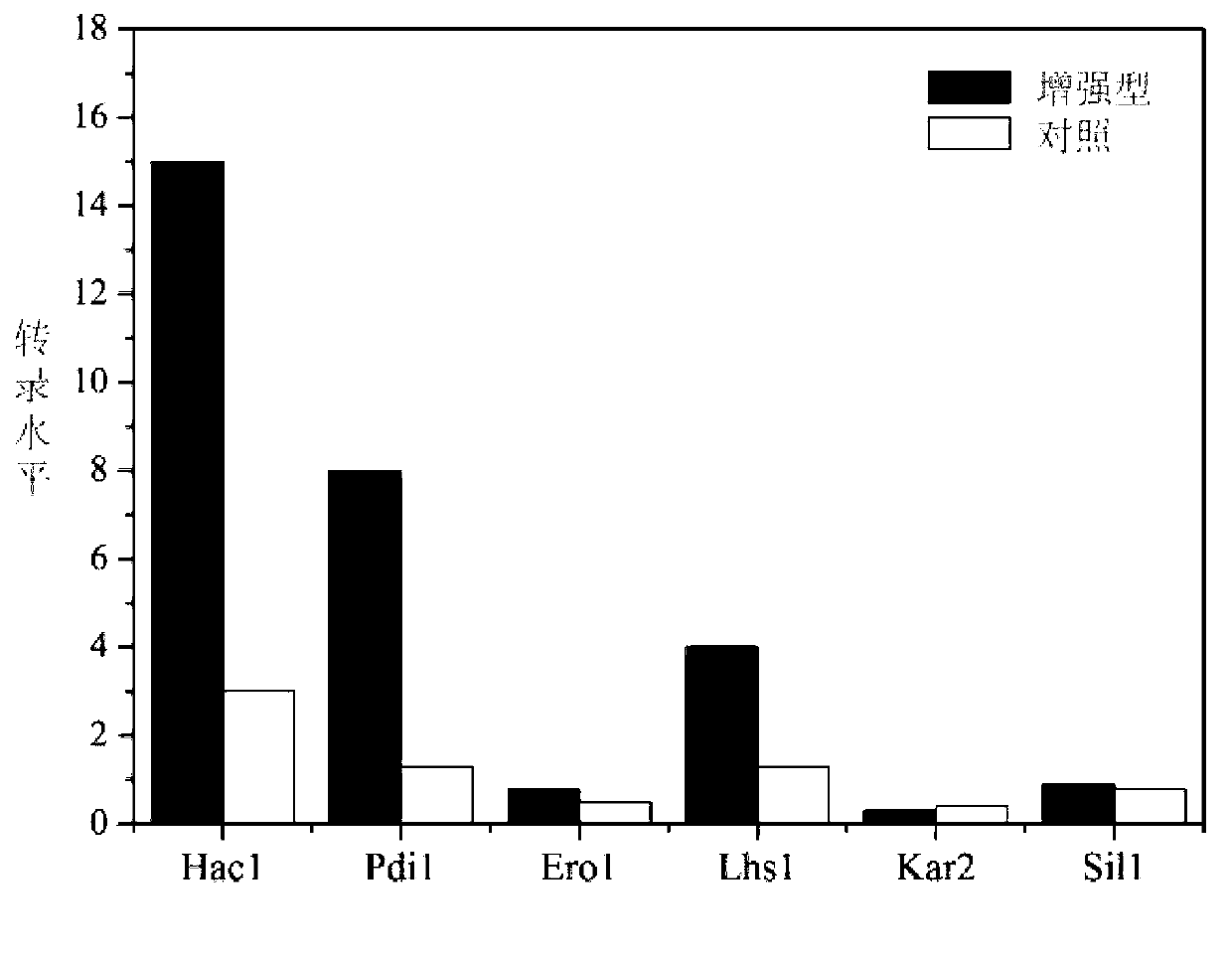
[0059] Example 2 RT-PCR determination of key secretion genes of secretion-enhanced strains and the influence on the physiological activities of the strains.
[0060] Medium: YPD medium (1L) for seed and slant medium: tryptone 20g, yeast extract 10g, glucose 20g; slant medium with agar 20g; basic fermentation medium is BMGY medium (1L): tryptone 20g, yeast extract 10g, glycerol 10mL, YNB 13.4g, 100mM phosphate buffer (pH 6.0); induction medium is BMMY medium (1L): tryptone 20g, yeast extract 10g, methanol 8mL, YNB 13.4 g, 100mM phosphate buffer (pH 6.0);
[0061] Cultivation method: Cultivate to OD at 30°C and 200rpm 600 The seeds between 1.6 and 1.7 are transferred to the basic fermentation medium with an inoculum of 2%, and cultivated at 30°C and 200rpm;
[0062] Induction conditions: when cultured in BMGY to an OD value of 1.2-1.5, the yeast cells were transferred into BMMY medium to induce protein production.
[0063] One day after the induction of the secretion-enhanced...
Embodiment 3
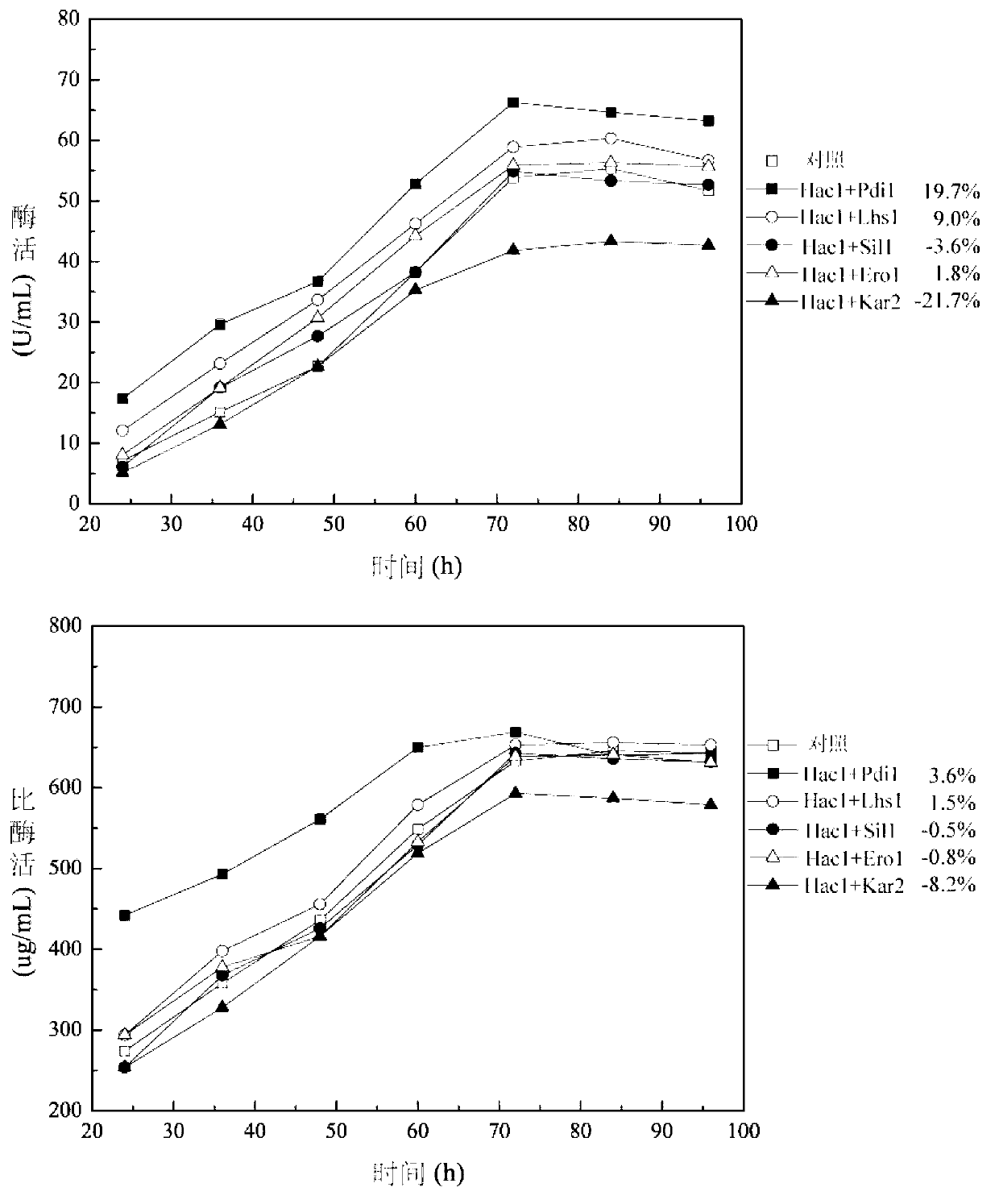
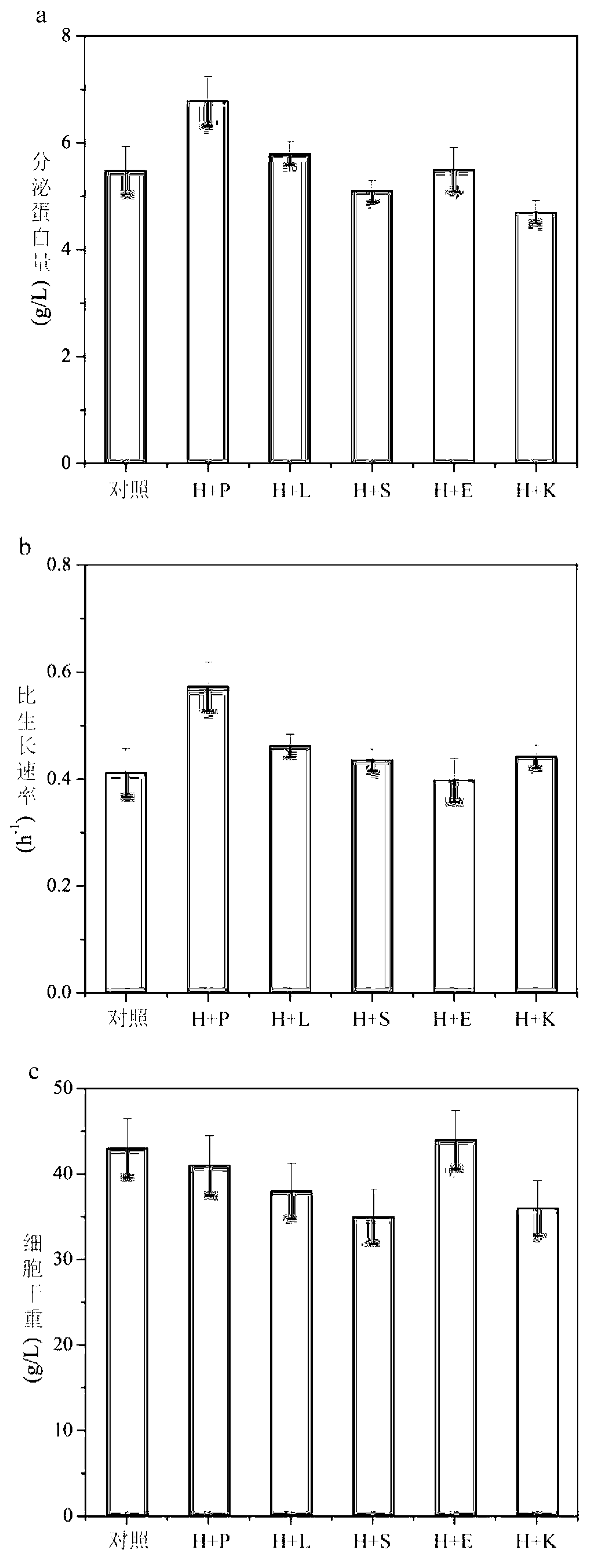
[0077] Fermentation of embodiment 3 secretion-enhanced bacterial strain on 3L fermentor
[0078] Pichia pastoris GS 115-pPIC9K-GOD / pPICZ-Hac1 / pPIC3.5K-Pdi1 was used as the starting strain.
[0079] The cultured YPD bacterial solution was aseptically inserted into a 3L fully automatic fermenter (LiFlus GMBioTRON, Korea) with an inoculum size of 10%. The initial conditions are: liquid volume 800-1000mL, initial stirring speed 500r / min, ventilation volume 2.5vvm, 30% phosphoric acid solution and 25% concentrated ammonia water to control the pH to 5.5, and the culture temperature during the growth period is 30°C , using DO-stat control associated with stirring to maintain DO at 30%, and set the maximum stirring speed to 950r / min.
[0080] When glycerol is exhausted (DO rises rapidly), and DO>60%, start to add 50% glycerol medium in different exponential feeding ways. When glycerol is exhausted again, DO rebounds again, and when DO>60%, induction begins. Reduce the induction tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com