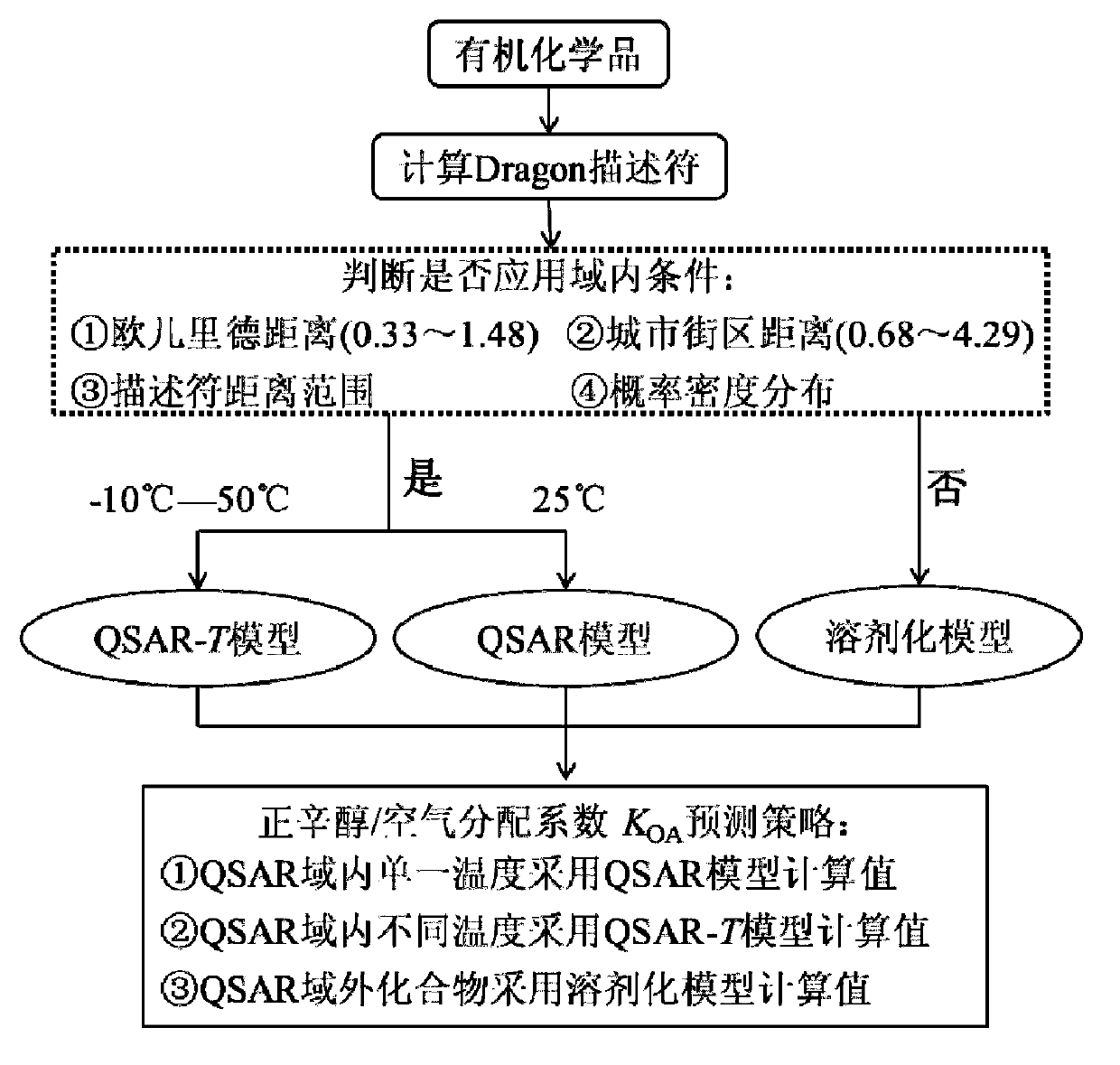
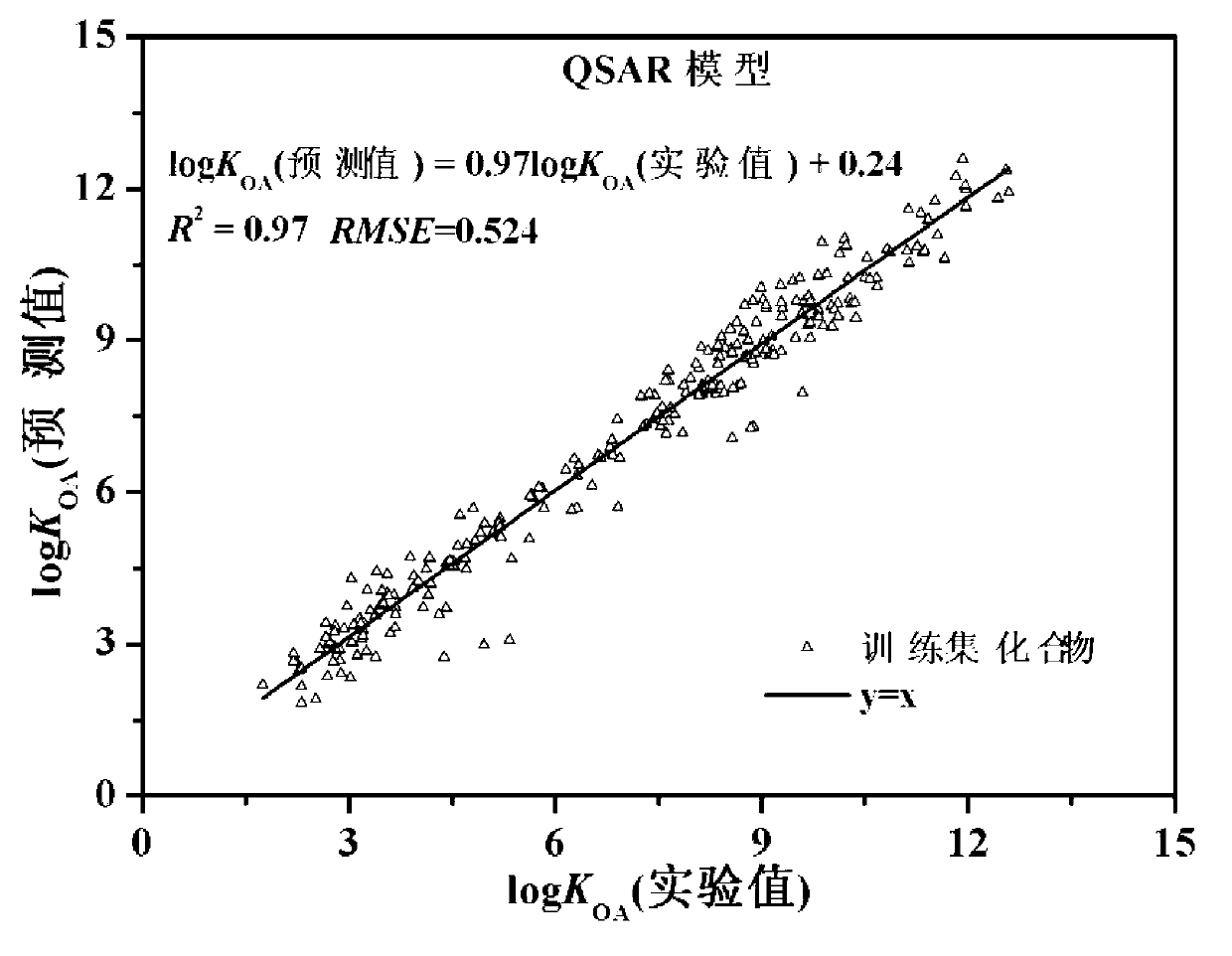
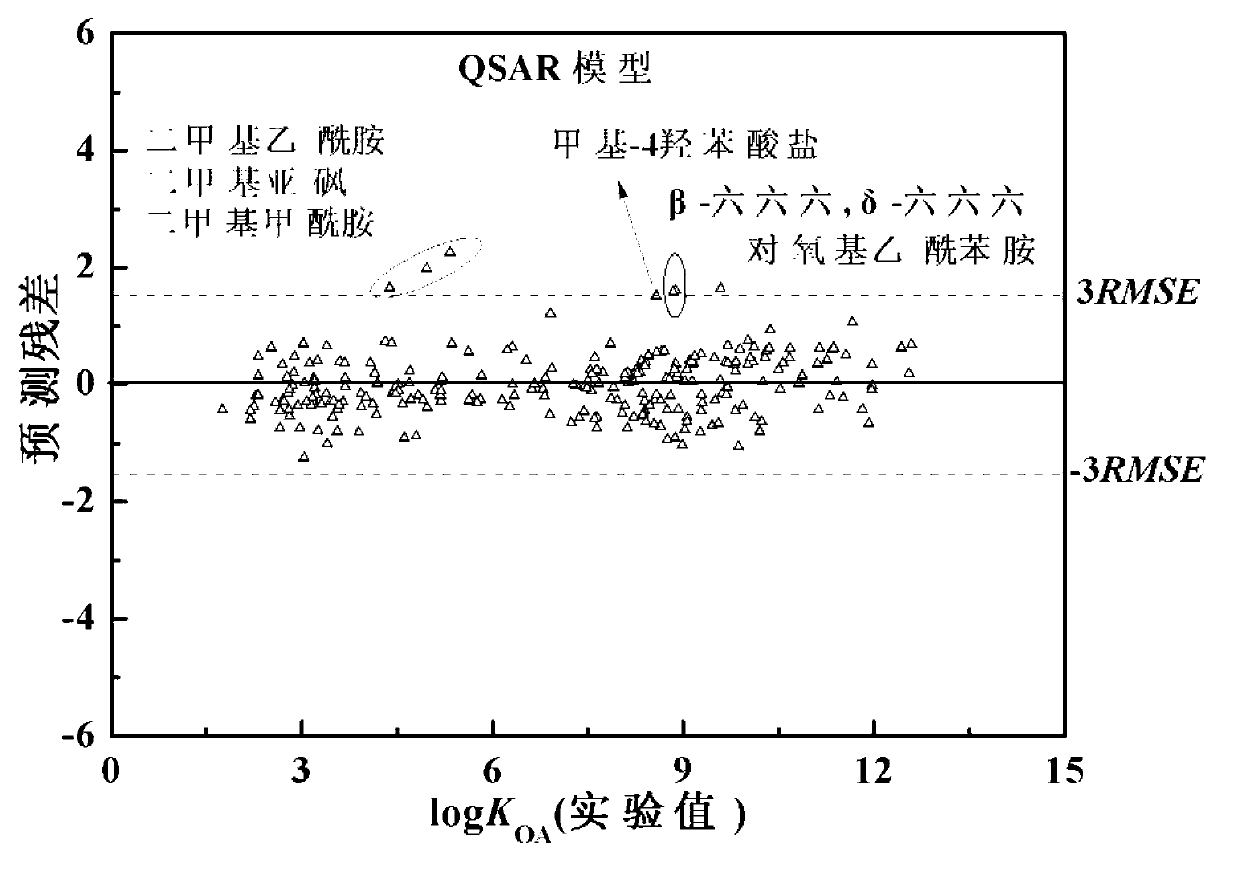
Method for predicting n-octyl alcohol air distribution coefficient (KOA) at different temperatures through quantitative structure-activity relationship and solvent model
A technique for quantitative structure-activity relationship and model prediction, which is used in electrical digital data processing, special data processing applications, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Using the method of the present invention to predict the n-octanol / air partition coefficient K of a biphenyl compound-polychlorinated biphenyl PCB-66 (2,3',4,4'-tetrachlorobiphenyl) at 25°C OA . The prediction method is:
[0046] (1) First, use chemical mapping software to obtain and save PCB-66 as a .mol format file. Use MOPAC software to conduct preliminary structural optimization; (2) Calculate 11 kinds of Dragon descriptors in the QSAR model by Dragon software, and the results are: X1sol=8.73, Mor13v=-0.33, H-050=0, R5v=0.118, T(O..Cl)=0, HATS5v=0.254, RDF035m=0.443, RCI=1.48, n COOR =0, Mor15u=0.155, RDF090m=0.334; (3) Judgment according to the judgment method of the application domain: ① Descriptor distance: The descriptor distance determined by the compounds in the training set is X1sol~(1,13.35), Mor13v~(- 1.538,0.938), H-050~(0,1,2), R5v~(0,0.319), T(O..Cl)~(0,100), HATS5v~(0,1.115), RDF035m~(0, 59.03), RCI~(0,1.54), n COOR ~(0 or 1), Mor15u~(-1.25, 1.75...
Embodiment 2
[0050] The n-octanol / air partition coefficient values of a polychlorinated naphthalene compound 1,4,6,7-tetrachloronapthalene at 283.15K, 293.15K, 298.15K, 303.15K, 313.15K, and 323.15K are predicted by the method of the present invention and compared with the experimental value. K of the compound at different temperatures using the QSAR-T model OA Make predictions. The prediction steps are as follows:
[0051] First, use chemical mapping software to obtain and save 1,4,6,7-tetrachloronaphthalene as a .mol format file. Using MOPAC2000 software for preliminary structure optimization. Calculate X1sol, Mor13v, H-050, R5v, T(O..Cl), HATS5v, RDF035m, RCI, n by Draogon software COOR , Mor15u and RDF090m. Then judge the QSAR application domain for these 11 descriptions, and determine that the target compound is in the application domain of the QSAR-T model. Then divide the calculated value of the descriptor by the corresponding temperature to obtain the values of the 11 d...
Embodiment 3
[0056] Given a diphenyl ether compound methoxy polybrominated diphenyl ether 6-OH-BDE-157. Also calculate its 11 kinds of Dragon descriptors, the values are X1sol=12.77, Mor13v=-0.921, H-050=1, R5v=0.103, T(O..Cl)=0, HATS5v=0.245, RDF035m=84.685, RCI=1.41,n COOR =0, Mor15u=1.33, RDF090m=14.19. Judgment according to the judgment method of the application domain: ① Descriptor distance: The descriptor distance determined by the training set compound is X1sol~(1,13.35), Mor13v~(-1.538,0.938), H-050~(0,1,2 ), R5v~(0,0.319), T(O..Cl)~(0,100), HATS5v~(0,1.115), RDF035m~(0,59.03), RCI~(0,1.54), n COOR ~(0 or 1), Mor15u~(-1.25, 1.75), RDF090m~(0, 53.63). Among the 11 descriptors, RDF035m is outside the scope of the descriptor, so the compound is outside the domain; ②Euclidean distance: the calculated Euclidean distance value is 0.829, which is between (0.33-1.48);③ City block distance: the calculated city block distance value is 2.09, which is within (0.68~4.29); ④ probability de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com