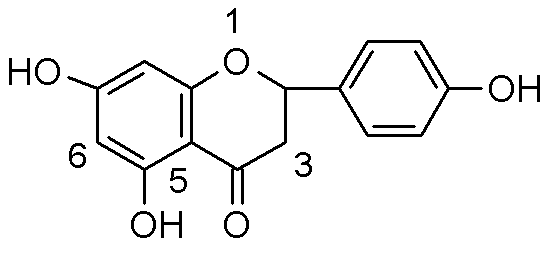
6,8-substituted naringenin derivative and application thereof
A Derivative, Naringenin Technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
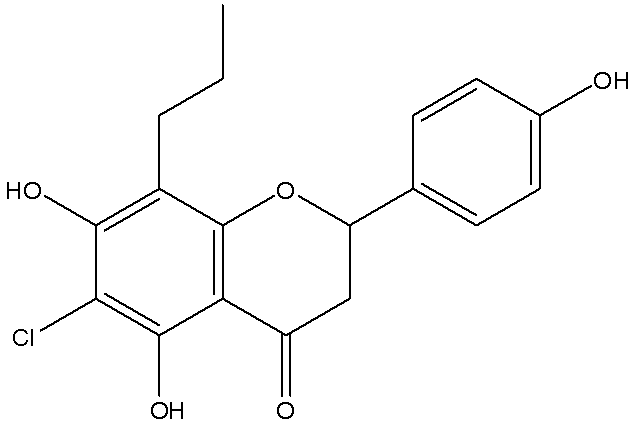
[0049] Example 1: 8-n-propylnaringenin (8-Propyl-2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one).
[0050]
[0051] 1 g of 8-allylnaringenin (compound 18) was dissolved in 100 ml of ethanol. Add 10% Pd / C 0.5g hydrogenation at normal pressure for 20 hours. After suction filtration, the filtrate was concentrated and purified by silica gel column chromatography with petroleum ether: ethyl acetate = 2:1 to obtain 0.4 g of a yellow solid. The melting point is 195-197°C; thin-layer chromatography was carried out with petroleum ether: ethyl acetate = 1:1 as the developing solvent, Rf = 0.6; mass spectrum LRMS M-H = 313.4, HPLC purity = 99%, yield = 40%.
[0052] NMR: 1 H-NMR (400MHz, DMSO-d6): δ0.872 (3H, t, J=7.2Hz; H-3″), δ1.441 (2H, q, J=7.2 Hz, H-2″), δ2 .415(2H,t,J=8.4Hz,H-1〃),δ2.651(1H,dd,J 1 =16.8Hz,J 2 =2.8Hz,H-3a),δ3.213(1H,dd,J 1 =12.8Hz, J 2 =16.8Hz,H-3b),δ5.386(1H,dd,J 1 =12.8Hz,J 2 =2.8Hz, H-2), δ5.945(1H, s, H-6), δ6.788 (2H, d, J=8.8 Hz, H-3′, H...
Embodiment 2
[0054] Example 2: 6-n-propylnaringenin (6-Propyl-2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one).
[0055]
[0056] 1 g of 6-allylnaringenin (compound 19) was dissolved in 100 ml of ethanol. Add Pd / C 0.2g and hydrogenate at normal pressure for 20 hours. After suction filtration, the filtrate was concentrated and purified by silica gel column chromatography with petroleum ether: ethyl acetate = 3:1 to obtain 0.4 g of a light yellow solid. The melting point is 173-175°C; thin-layer chromatography was carried out with petroleum ether: ethyl acetate = 1:1 as developing solvent, Rf = 0.6; mass spectrum M-H = 313.2, HPLC purity = 96%, yield = 13%.
[0057] NMR: 1 H-NMR (400MHz, DMSO-d6): δ0.822 (3H, t, J=7.2Hz; H-3″), δ1.417 (2H, q, J=7.2Hz, H-2″), δ2 .397(2H,t,J=7.2Hz,H-1〃),δ2.733 (1 H,dd,J 1 =17.2Hz,J 2=3.2Hz, H-3a), δ3.162(1H,dd,J 1 =12.4Hz,J 2 =16.8Hz, H-3b), δ5.408(1H,dd,J 1 =12.4Hz,J 2 =2.8Hz,H-2),δ5.976(1H, s,H-8),δ6.801 (2H,d,J=8.4Hz,H-3′,H-5′),δ7.304...
Embodiment 3
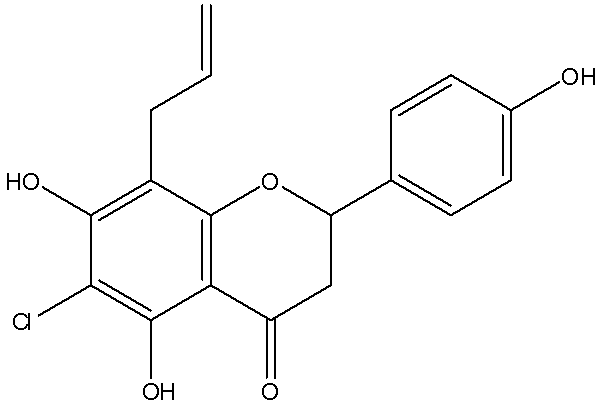
[0059] Example 3: 6-bromo-8-allylnaringenin (8-Allyl-6-bromo-2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one) .
[0060]
[0061] Dissolve 0.7 g of 8-allylnaringenin (compound 18) in 50 ml of carbon tetrachloride (CCl 4 ), add 2 ml dimethylformamide, add 1.05eq N-bromosuccinimide (NBS), and heat to reflux for 5 hours. After cooling and concentrating, the sample was directly mixed and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 0.48 g of a light yellow solid. Melting point is 203-205°C; TLC with petroleum ether: ethyl acetate = 3:1 as developing solvent, Rf = 0.6; mass spectrum LRMS: M-H = 391.2, 389.2; HPLC purity = 89.22%, yield 55% .
[0062] NMR: 1 H-NMR(400MHz,DMSO-d6):δ2.82(1H,dd,J 1 =2.8 Hz, J 2 =17.2Hz, H-3b), δ3.275(3H,m,H-1″,H-3a),δ4.901(2H,m,H-3″),δ5.478(1H,dd, J 1 =2.8Hz,J 2 =12.8Hz,H-2),δ5.795(1H,m,H-2〃),δ6.800(2H,t,H-3′,H5′),δ7.305(2H,d,J= 8.8Hz, H-2′, H-6′), δ9.553(1H, s, H-4′OH), δ10.334(1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com