N-(4-fluorobenzyl)-3, 5-bis(4-trifluoromethyl benzylidene)-4-piperodone and preparation as well as application
A technology for trifluoromethylbenzylidene and trifluoromethylbenzaldehyde, which is applied in the field of chemical drugs, can solve problems such as weak selectivity, and achieve the effects of low acute toxicity in vivo and small toxic and negative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of embodiment 1N-(4-fluorobenzyl)-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone
[0032] In an ice-water bath at 0°C, add 0.4 mol of methyl acrylate and 7 mL of methanol into a 100 mL three-neck flask, and after stirring for 30 min, slowly add 0.1 mol of 4-fluorobenzylamine and 10 mL of methanol dropwise to control the rate of addition. , so that the system temperature does not exceed 50°C (about 1 drop / second). After the dropwise addition was completed, stir at room temperature for 30 min, continue to raise the temperature to 65° C., reflux, and track the reaction progress by thin layer chromatography (TLC). After reacting for about 8 hours, the reaction was stopped, and methanol and unreacted methyl acrylate were recovered by distillation under reduced pressure to obtain light yellow oily liquid intermediate (b).
[0033] At room temperature, add 15mL of anhydrous toluene and 0.1mol of sodium metal (cut into blocks) into a 250mL dry three-...
Embodiment 2
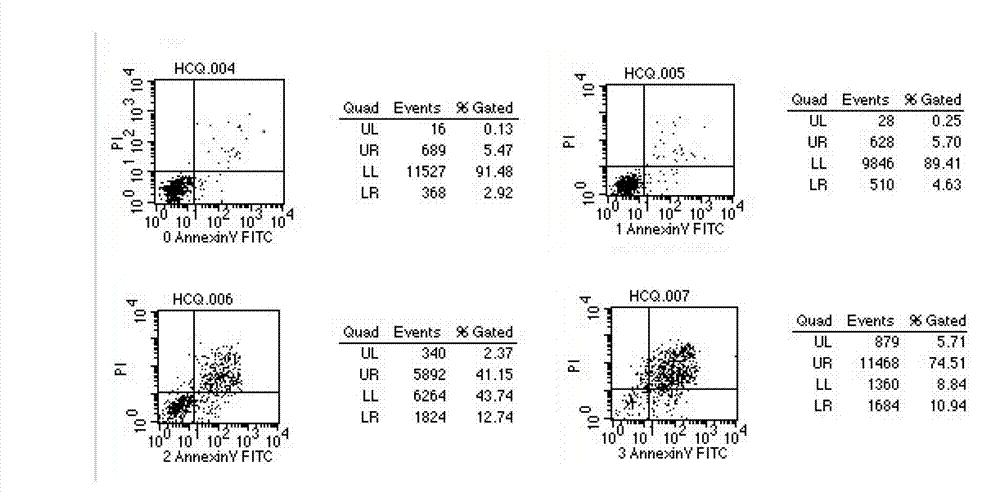
[0039] Example 2 Compound ALB65 inhibits the proliferation of 12 leukemia cancer cell lines including leukemia K562 IC 50 the test
[0040] 1. Test reagents and equipment
[0041] Experimental drugs and reagents: self-made compound ALB65, dissolved in DMSO, added distilled water to the required concentration (DMSO concentration ≤ 1‰), sterilized and stored at 4°C. MTT (tetramethylazolazolium blue) reagent was purchased from Sigma. Human leukemia cells K562, Jarket, U937, THP-1, NB 4 BF, MM-6, HK-2, HL-60, ML-2, KG-1a, Molm-13, SP2 / 0 cell lines were purchased from Shanghai Chinese Academy of Sciences Cell Bank. 10% SDS reagent (Sino-American Biotechnology product), RPMI-1640 (GiBCo, USA) culture medium containing 20% fetal bovine serum (FBS) was used, and other reagents were commercially available analytically pure. At 37°C, 5% CO 2 Subculture was carried out in an incubator with saturated humidity, and the cells were used for experiments when they were in the logarithmi...
Embodiment 3
[0050] Example 3 Compound ALB65 inhibits the proliferation of ovarian cancer, breast cancer, liver cancer and esophageal cancer cell lines IC 50 the test
[0051] 1. Test reagents and equipment
[0052] Experimental drugs and reagents: self-made compound ALB65, dissolved in DMSO, added distilled water to the required concentration (DMSO concentration ≤ 1‰), sterilized and stored at 4°C. MTT (tetramethylazolazolium blue) reagent was purchased from Sigma. Human leukemia cells, ovarian cancer HO-8910 cell line, breast cancer MCF-7 cell line, liver cancer SMMC-7721 cell line and esophageal cancer ECA-109 cell line were purchased from the Cell Bank of Shanghai Chinese Academy of Sciences. 10% SDS reagent (Sino-American Biotechnology product), RPMI-1640 (GiBCo, USA) culture medium containing 20% fetal bovine serum (FBS) was used, and other reagents were commercially available analytically pure. At 37°C, 5% CO 2 Subculture was carried out in an incubator with saturated humidity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com